Computer-Based Modeling & Simulation: Not Just a Tool for Quicker Product Approval
Computer-based modeling and simulation are increasingly important during the development and authorization of medical devices and for a lot of manufacturers’ market success.
This article:
- Will give you an overview of the possibilities offered by modeling and simulation
- Details the hurdles and regulatory requirements
- Identifies links to the most important sources
- Gives specific tips for use for medical device manufacturers
1. What is meant by computer-based modeling & simulation?
a) Definition of terms
A useful definition of the terms “computer-based modeling” or “computational modeling” comes from the FDA itself:
Computational modeling is the process of representing a real-world system by means of a computer and then running the simulation by implementing a numerical scheme.
Frontiers in Medicine, September 2018, Volume 5, Article 241 by Tina Morrison (FDA) et. al.
One of the possible applications of such modeling and simulation is “in silico medicine.” This can be defined as follows:
It is the direct use of computer simulation in the diagnosis, treatment, or prevention of a disease. More specifically, in silico medicine is characterized by modeling, simulation, and visualization of biological and medical processes in computers with the goal of simulating real biological processes in a virtual environment.
b) Modeling and simulation in other industries
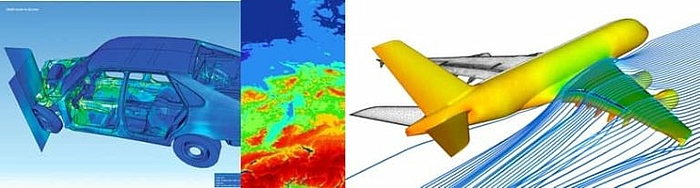
The use of computer models and simulation is widespread in other industries:
- Car manufacturers simulate crash tests. They use crash tests with real cars primarily for the validation and enhancement of computer models.
- The development of aircraft and the testing of their flight characteristics is initially carried out exclusively on computers.
- The weather forecast is based on computer models.
- Manufacturers calculate the mechanical properties of new products using methods such as the finite element method.
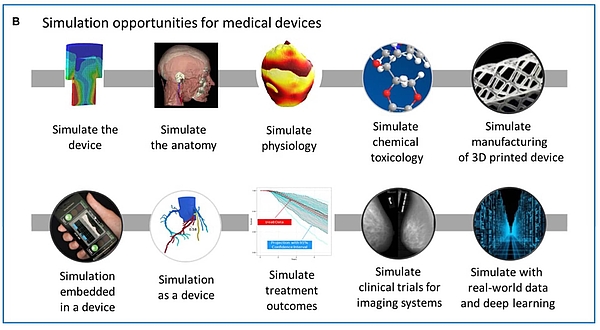
2. Application of computer-based modeling in medical technology
Computer-based modeling and simulation can be used by medical device manufacturers in all phases of a device's life cycle.
a) Development of medical devices
Manufacturers must justify the “solution chosen”
Laws such as the MDR and the IVDR require manufacturers to comply with the general safety and performance requirements. These include requirements for
- The mechanical, electrical, electromagnetic and biological safety of the devices
- Reliability and first-failure safety
- Compliance with the performance characteristics
Manufactures must justify why they have chosen a particular solution, e.g. a specific design:
“The documentation shall […] include a justification […] of the solutions adopted to meet those [general safety and performance] requirements.
MDR, Annex II, Chapter 4.
This justification will be particularly successful if manufacturers can demonstrate that other solutions are inferior to the chosen solution.
Modeling and simulation can be used for this
A very efficient way of doing this is to simulate alternative solutions on a computer and select the best option. These options involve the:
- Selection of materials
- Design of components, e.g., shape, material thickness
- Design and positioning of antennas
- Design of user interfaces
b) Production
Some manufacturers use computer-based modeling to simulate production processes, e.g.:
- Filling of products
- Sterilization and disinfection
- Design of measuring systems
c) Verification, validation and authorization
The evidence that a medical device is safe and effective and provides the promised clinical benefits can be generated in part or in full using computer models.
In some cases, the FDA has even made a decision on the authorization of medical devices based exclusively on such models and simulations. This includes a device whose “MRI compatibility” had to be proven.
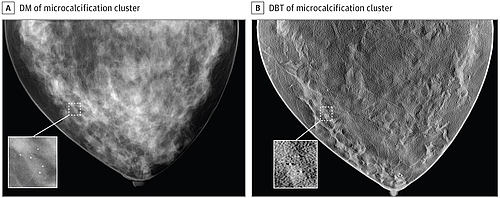
In addition, the FDA has authorized a device for “3D mammography” whose diagnostic performance was evaluated using synthetic(!) images. For this, the manufacturer simulated both the breast (e.g. size, fat content, geometry, mammary ducts, vessels, lesions) and the medical device itself.
As a result, a large part of the clinical investigation was performed by simulation with in-silico clinical trials.
d) Post-market phase
Simulation can also help manufacturers in the post-market phase:
- Error patterns in the field can be simulated using computer models and the causes identified
- Manufacturers can quickly try different troubleshooting measures and select the best option.
- Models can also be used to verify and validate these measures and prove their effectiveness to authorities.
- Models help to predict future errors. They also allow manufacturers to simulate extreme situations.
e) Summary
There is hardly a phase in the device life cycle in which modeling and simulation cannot be used. The FDA agrees.
3. Advantages (not just) for medical device manufacturers
a) Examples
The advantages of computer-based modeling and simulation are obvious:
- The speed of development can be increased because there is no need to develop physical prototypes.
- Devices are safer because different options can be explored and the best option chosen.
- The devices can also be tested under extreme conditions, further increasing their safety.
- Animal experiments can be minimized or even avoided completely.
- Expensive benchtop test can be, in part, replaced by computer models.
- The costs and duration of clinical studies can be minimized.
- Fewer patients have to participate in clinical studies.
- An expansion of the patient cohort to include synthetic patients is possible.
b) Summary
Computer-based modeling offers the following advantages:
- Shorter time to market
- Lower costs
- Safer devices
- Fewer ethical conflicts as a result of animal testing and clinical investigations
But these methods also have their price. More on this below.
4. Regulatory requirements for computer-based modeling & simulation
a) Europe
Even up-to-date laws, such as the MDR and IVDR, do not contain specific requirements for the use of computer models for the development of medical devices. However, they explicitly provide for their use and simulation.
where appropriate, the results of biophysical or modelling research the validity of which has been demonstrated beforehand; (Annex I, 10.1a))
results of tests, such as engineering, laboratory, simulated use and animal tests [...]; (Annex II, 6.1.a))
the pre-clinical testing, for example laboratory testing, simulated use testing, computer modelling, the use of animal models (Annex VII, 4.5.4.a))
ISO 13485 requires manufacturers to:
- Validate their computerized systems
- Validate their processes
- Validate measuring equipment
- Plan monitoring and measuring processes
- Use appropriate statistical methods
b) USA/FDA
The FDA was mandated by the US Congress to promote and regulate the use of computer-based modeling and simulation. It had already published the document “Advancing Regulatory Science at FDA” in August 2011. In it, the authority chose to focus on the “use and develop computational methods and in silico modeling.”
In 2018, Tina Morrison, who is driving the issue forward at the FDA, explained in an article in “Frontiers in Medicine” the possibilities offered by “computational modeling for medical devices” and the required “regulatory science.”
Even before that, she had played a key role in the development of the guidance document “Reporting of Computational Modeling Studies in Medical Devices Submission”, a final version of which has been available since 2016.
As the name suggests, this document describes what documentation the FDA expects. But it does not contain very specific guidelines for the validation of the models.
c) ASME V&V 40
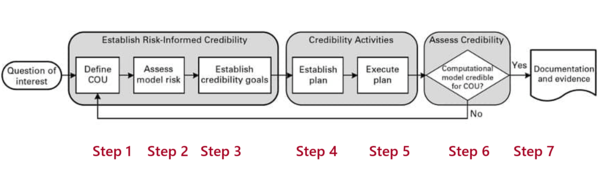
For this, the FDA relies on the “V&V 40” published by the American Society for Mechanical Engineering (ASME) with the title “Verification and Validation of Computational Modeling of Medical Devices”.
The document describes the steps manufacturers should take when validating computer models.
These steps include:
- Formulating the question of interest
Specify exactly what the question to be answered by the model is. For example, how much fluid should I remove from dialysis patients? - Describing the context of use (COU)
The COU describes what role the computer model has in answering the question and what the specific object of the computer model is. For example, it will simulate the human fluid compartment of a patient in order to estimate the hydrogenation. - Analyzing the risks
The risks are determined by the influence of the model on the manufacturer's decision and the consequences of this decision. For example, the influence of the model would be greater if the manufacturer relied solely on the model. - Establishing the “credibility goals”
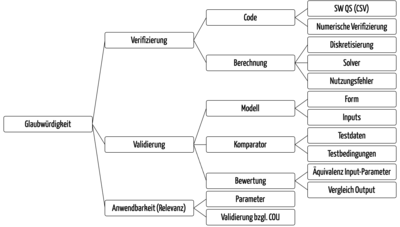
Here, the ASME document describes numerous aspects (see Fig. 7) for which manufacturers must define criteria. These range from the analysis of discretization errors to the adequacy of the model and its formulas and constants through to the verification of the comparator used to validate the model.
5. Use of computer-based modeling and simulation in practice
a) Challenges
The advantages of computer models for the development and authorization of medical devices are clear. However, in practice manufacturers are faced with numerous challenges:
- Shortage of experts
Experts capable of developing computer models and simulating medical devices are rare and difficult to recruit. - Regulatory uncertainty
Because the European authorities and notified bodies are not driving the regulatory science forward to the same extent as they are in the US, there is still some regulatory uncertainty. Will the simulations and the validation of the models be recognized? How much other evidence is expected? - High cost of the tools
There are many open source tools. But they usually have a very limited scope of application. Furthermore the support for the validation of these tools is missing, and the usability leaves wishes open. Professional tools on the other hand can be extremely expensive. - The time required to develop and validate the models
The development and validation of computer models is very time consuming. For example, it took 1.75 years for the 3D mammography model. However, if the model hadn’t been used, it would have taken four years.
b) Taking the next steps
As with any venture into the unknown, we recommend the following best practices:
- Start with clear and very limited questions.
- Employ people who have at least some experience in the topic and who enjoy the project.
- Get help, e.g., in the form of advice and training
- Learn quickly from mistakes and adapt questions, methods and tools.
- Don’t reinvent anything, use what already exists: service providers like Cadfem Medical offer “simulation as a service” with (pre-)validated models of devices and people (organs, anatomy).
- Use the FDAs open mindset and offer to follow Tina Morrisions (FDA) recommendation to use CM&S for approvals.
6. Summary
a) Almost no alternative – because of new competitors as well
In the long run, it may no longer be a question for most manufacturers of whether they use computer-based modeling and simulation for the development, validation and authorization of their devices or not.
Without these methods they will not be able to keep up with the competition. And the competition may not necessarily be their current competitors. Companies and universities with their start-ups who have modeling and simulation tools and expertise will enter the market.
b) A question of ethics
But it is not just competitive pressure that should prompt manufacturers to set out down this path. Exposing patients and animals to unnecessary tests and risks is simply unethical.
The FDA also sees opportunities here:
The all–in silico approach for conducting imaging trials is not intended to replace but rather complement or minimize traditional clinical trials. Incrementally incorporating computational results in regulatory submissions can, for example, help decrease the human trial size and length.
c) Manufacturers, authorities and notified bodies are required
The extent to which Europe is lagging behind many US initiatives when it comes to the regulatory science regarding computer-based modeling and simulation is worrying. More engagement from authorities and notified bodies with this issue is desirable.
Manufacturers can get involved with the Avicenna Alliance in order to actively help shape the regulatory environment.
The Johner Institute can provide even more intensive support for achieving regulatory clarity and making modeling and simulation as fast and easy to use as possible.