Recycling and Disposal of Medical Devices: How “Trash” Can Give You a Market Advantage
A lot of manufacturers leave the recycling and disposal of medical devices to operators such as hospitals. But this last phase of the device life cycle offers manufacturers new opportunities: from differentiation to cost savings to new business models.
Hospitals are also interested in new strategies due to economic pressures resulting from the ongoing pandemic and the increasing amounts of waste they have to dispose of.
An article by and with Professor Werner Lorke
1. Recycling and disposal: a conflict of objectives in the case of medical devices
High-quality materials...
Manufacturers are increasingly using high-quality materials, such as metals, plastics and combinations of the two, for example, in electronic components, because these high-quality materials make medical devices very safe and improve their performance.
For example, they have been proven to make devices very:
- Stable against mechanical and thermal loads
- Easy to clean, disinfect and sterilize
- Biocompatible
- Reproducible in terms of production
… should be re-used ...
It is precisely because these materials are of such high-quality that it would be better to re-use, recycle, or even refurbish them rather than sending them to landfill or incinerating them.
Reuse and refurbishment them helps:
- Conserve natural resources
- Minimize the environmental impact of the waste
… but not cause any risks
In the case of medical devices, however, there is a conflict of objectives:
- On the one hand, we want to protect the environment.
- On the other, it is important to protect people from infectious material and sharp objects.
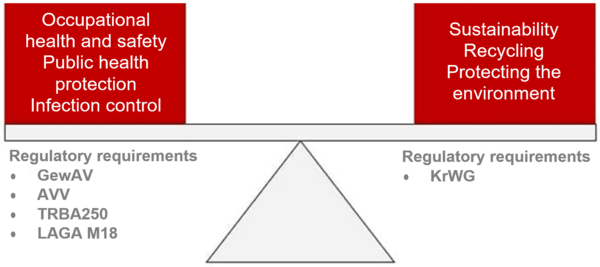
The primacy of hygiene is intended to protect patients, hospital and clinic staff and everyone else who comes into contact with the material being disposed of. As a result, the handling of used, infectiously or non-infectiously contaminated medical devices in hospitals and clinics is subject to regulations that give priority to occupational public health protection and occupational health and safety.
Tip
This article will give you an introduction to all the regulatory requirements regarding both the environment and occupational health and safety.
Sharps (injection cannulas, scalpels, curettes), for example, are considered hazardous waste and, therefore, have to be collected separately from other waste in special, type-tested containers. Provided that the containers remain securely closed, this waste can then be mixed with non-hazardous clinical waste, which can then be disposed of in waste-fed heating and power plants with no other special requirements.
This conflict of objectives is also evident in the regulatory requirements: the Kreislaufwirtschaftsgesetz aims to encourage recycling while the Gewerbe-Abfallverordnung is intended to protect public health and occupational health and safety.
2. Options for handling medical devices
a) Incineration and landfill
The easiest option is to incinerate the devices. “Waste to energy” is the euphemistic description. This has the advantage that all potentially dangerous germs and other biogenic contaminants on waste from healthcare facilities are rendered harmless.
Since large-scale thermal treatment plants are not yet the rule, particularly in the new eastern EU member states, and since the landfill ban applies throughout the European Union, complying with the regulations on the disposal of clinical waste in these countries often requires the waste to be transported long distances.
b) Reusing materials
A value-preserving separation of used medical devices so materials can be recycled would help reduce this waste tourism. However, to do this, it must be possible to separate and re-use the materials. For example, steels in surgical instruments and explants would have to be melted down.
Tip
Read more on the opportunities and limitations of waste reprocessing in the “Recycling, disposal and refurbishment” section.
c) Refurbishment
The third option is to refurbish the devices. However, this is only possible for a limited number of devices as refurbishment must “ensure that the reprocessed medical device does not pose any risk of damage to health during subsequent use, particularly with regard to – infections, – pyrogen-induced reactions, – allergic reactions, – toxic reactions – or due to altered technical or functional properties” (RKI, 2012).
This means that this option is only cost-effective for relatively high-priced devices, such as endoscopes and catheters. Reuse also requires that:
- The devices are made of a small number of different materials
- Their design allows them to be easily disassembled into their individual components
3. Recycling, disposal and refurbishment
a) Electrical medical equipment
In the EU, manufacturers generally have take-back system for large medical equipment (MRI scanners, CT scanners, x-ray machines, etc.) that has become obsolete. Some of these machines are then technically renovated and resold on the second-hand market. However, most of them are dismantled and recycled in accordance with the Abfallverordnung.
Small devices, instruments and surgical accessories whose repair is no longer cost-effective are either disposed of on an ongoing basis in clinical waste or returned bundled together as e-waste.
Unfortunately, this material is often not optimally recycled due to big differences in how much of it can be recycled and for economic reasons, e.g., raw material prices.
b) Simple disposable surgical instruments
Disposable instruments form their own special category of recyclable materials. In terms of quantity, “ward instruments” dominate. Ward instruments are simple surgical instruments made of chrome steel, for example, forceps, scissors, clamps and needle holders. Today, over 20 million such instruments are used in Germany per year.
These instruments are usually continuously disposed of in general clinical waste and consequently initially “incinerated”. Due to their size and magnetic properties, they could, theoretically at least, be separated from the grate ash.
A large part of the medical devices that will be in class I when the MDR comes into effect in May 2021 are currently explicitly designed as disposable devices.
There is the subclass Ir (r stands for “reusable”) but – for financial and hygiene reasons – there is a clear trend towards using a device on one person only.
c) Complex disposable instruments
Class IIb complex disposable instruments include, for example, multipolar electrophysiology (EP) catheters used in intracoronary operations.
Provided these instruments have been designed accordingly, the parts that have been in direct contact with the patient can be easily separated and recycled separately. The increased use of such hybrid instruments, which consist of reusable and disposable components, makes their refurbishment economically viable without reducing patient safety.
d) Instrument carriers, trays
The instruments, devices and endoprostheses required for a specific surgical operation are grouped together in trays and special instrument carriers by the central sterile supply department (CSSD). These containers consist of combinations of stainless steels, aluminum or titanium alloys and various plastics.
Unlike basic trays, which are usually universal, many of these trays are designed for specific products from one manufacturer. These special trays often become obsolete when a system is changed or new surgical techniques are introduced.
Since standard trays can also wear out through use, they ultimately end up as waste with a high material value.
e) Implants, explants
In addition to the stainless steels used in surgical equipment, sites that perform surgery, in particular, accumulate stocks of explants over time that should be recovered after the prescribed storage period. These medical devices consist mainly of metal alloys (titanium, cobalt-chromium, zirconium, stainless steels) as well as ceramics and plastic polymers.
The latter can only be separated with mechanical or thermal force. However, there are strict limits to commercial recovery.
For example, although the energy requirements and the process costs for the production of primary titanium are very high, metallurgical recovery of titanium used for medical purposes is barely worthwhile. That's because the metal
- is used by manufacturers in different alloys,
- is bonded to other materials or
- contains, for example, holes that lead to defects in the secondary material in the vacuum melt.
This titanium can be used as an additive in steel production (ferrotitanium) instead.
As the other metals mentioned are easier to process through metallurgical smelting, higher raw material prices are paid for them. Cobalt-based alloys are of particular interest, as are nickel-containing steels.
f) Plastics
In medical devices that come into direct and, above all, prolonged contact with body fluids (e.g., dialyzers), biocompatibility is one of the key factors when the materials are selected. The use of a wide range of plastic polymers has become established:
Polyolefins (PE, PP), polyacrylates, and silicone and polyurethane compounds are the main ones, with the highly chemically inert polytetrafluorethylene (PTFE) as well as polyether ether ketones (PEEK) and polysulfones (PSU) used as well.
In other medical/clinical contexts, polystyrene is increasingly used instead of glass for infusate containers, and PVC is used for blood bags, for example.
g) Instruments for minimally invasive surgery
Minimally invasive surgery shortens recovery times and, therefore, the time patients spend in hospital. As a result, they are increasingly becoming the preferred treatment option in surgery, urology and cardiology, among others.
The basic instruments required for minimally invasive surgery include various forms of endoscopes optically, mechanically and electrically/electronically designed for tissue collection, heat treatment or electrophysiological stimulation.
EP catheters are used as disposable instruments in cardiology. They contain electronic components, plastics and a platinum electrode tip. The latter makes used EP catheters extremely attractive for waste disposal companies.
Products macroscopically containing different materials can, at least in principle, be separated thermally and/or mechanically. A targeted re-design can improve the economic viability of recycling the most valuable materials.
4. The current situation
a) A lot of manufacturers rely on single-use medical devices
A lot many manufacturers rely on disposable devices. This has several advantages for them:
- They save themselves the work of having to design the devices specifically for recycling or refurbishment.
- Risks specific to the refurbishment and re-use of materials are automatically controlled.
- The continual sale of new devices guarantees sales revenues.
- The special regulatory requirements for reusable (surgical) devices do not apply to disposable instruments.
b) Hospitals and clinics only make a small contribution to recycling
In order for the valuable materials to be recycled, they have to be separated from normal waste, as is the case with household waste. Hospitals and clinics also separate their waste.
However, this separation mainly involves consumer goods, such as glass, paper and cardboard, which are produced on a daily basis on a larger scale. In contrast, used sharps must be collected in special, securely sealed containers for disposal.
Because a lot of medical devices contain numerous materials, it’s not possible to assign them to one material class. In addition, it’s often impossible to tell what the components are just by looking at them.
Therefore, a demand for further separation of waste would be difficult to comply with given hospitals’ and clinics’ notoriously overloaded daily schedule. It would be impractical and also uneconomical.
c) The recycling of plastics is not economical
It is true that monolithic polymers, such as composite films can – at least in principle – only be separated in chemical-thermal-physical process cascades. In practice, however, a) this is usually completely uneconomical and b) there are no consumer markets for such secondary materials.
A typical example would be the polysulfone hollow fibers used in dialysis machine membrane filter cartridges. Polysulfones are very expensive materials. The development of material recycling processes would be particularly useful in this case, since the same polymers – albeit in much larger quantities than are used for apheresis – are also generated as waste in composite osmosis membranes used in seawater desalination plants.
d) Chemical recycling is difficult
For PTFE, which cannot be processed thermoplastically, there are chemical recycling plants that can generate the monomers TPE and HFP (hexafluoropropylene) from it.
Since highly toxic hydrogen fluoride (hydrofluoric acid, HF) is produced when fluorine-containing compounds are incinerated, they can only be incinerated in special plants. Therefore, the recycling of PTFE and its compounds also contribute to a reduction in environmental pollution.
The ultra-high-density polyethylenes (UHDPE, UHMWPE) used in prosthetic implants can be thermoplastically recycled but – as with PTFE – this changes its polymer structure, and the material loses important properties as a result.
There are established recycling processes for high-density polyethylene (HDPE), which has the same chemical composition. However, separate recovery while preserving the special chemical structure of UHDPE is not economically viable.
The other plastics mentioned above can also only be bundled to an extent that reprocessing them becomes profitable if they are separated well-enough at the point of origin or separated downstream by a waste disposal company.
Otherwise, the only option that remains is thermal recovery in waste-fed heating and power plants as the lowest-value use option.
e) Environmental awareness is increasing
Customers (i.e., physicians, nursing staff and patients) are increasingly aware of the global damage to the environment caused by waste and the finite nature of resources. The “throwing away” of medical equipment, including equipment explicitly intended for single use, is being viewed increasingly critically by users and the public.
f) Conclusion: there is a big need for optimization
There are no established recovery paths for recyclable clinical materials in Germany or Europe that take their complex high value content into account. Leaving metal-containing explants and instruments to a general scrap metal recycling company is difficult because of the organic material on them and, in addition, is usually not a solution for value-preserving recycling because of the challenging material combinations.
In the case of a lot of end-of-life medical devices, there is usually no high-quality recycling because:
- Public health requirements make it difficult
Due to the quantities involved, it is not worthwhile for individual healthcare operators and is associated with additional expense
5. Regulatory requirements for waste disposal
a) Overview
There are a lot of legal regulations in Germany for the disposal of used medical devices and the recovery of materials they contain:
- Occupational health and accident prevention
- General public health protection and infection control
- Waste legislation (EU Waste Framework Directive, Bundeskreislaufwirtschaftsgesetz, waste disposal legislation in the German Länder, municipal statutes)
- Transport law
Parts of the regulations mentioned above are relevant for collection and disposal processes at hospitals and clinics, others are relevant for processes outside of hospitals and clinics.
As is the case for medical device law, there is a legal hierarchy:
- EU law
- National laws
- Implementing regulations
- Other recommendations
However, these regulations are not always free of contradictions. In practice, this makes it difficult to work out and implement the measures required.
b) Occupational health and safety and infection control
Regulatory requirements
In the case of non-state institutions, the professional associations are responsible for statutory accident insurance. In the case of healthcare operators, the Berufsgenossenschaft für Gesundheitsdienst und Wohlfahrtspflege (BGW) establishes requirements for employers to ensure that the personnel are covered by insurance.
These requirements are, in turn, based on the TRBA 250 (Technical Rules for Biological Agents) regulations for the professional handling of biological agents in health care and welfare facilities.
There are also the recommendations (implementation tools) from the Länderarbeitsgemeinschaft Abfall for the handling of waste from healthcare operators (LAGA M18). This is based on the waste producer's classification of their own waste based on the to the Abfallverzeichnis-Verordnung (AVV).
Based on the waste codes (AS) in the AVV, a distinction is made between hazardous (AS with *) and non-hazardous waste from healthcare operators. The specifications for occupational health and safety are then derived from this.
Risk assessment
For this purpose, a hospital's/clinic’s entire waste management system is subject to a risk assessment. The employees’ activities must be evaluated with regard to the potential risks:
- In the wards: separate collection of contaminated and/or hazardous waste in containers
- During transportation in the hospital/clinic: Collection, interim storage
- During the final loading onto the waste disposal vehicles
This assessment must clarify the health risks associated with each activity, for example:
- Stab and cut injuries
- Infections caused by viruses or bacteria
- Physical loads (e.g., from transport work)
If these risks exceed the level of risk already present at the facility from its normal activities, appropriate actions must be defined and implemented. These include:
- Instructions on the procedure for collecting, transporting, storing and disposing of waste.
- Binding, adequate and repeated training on these instructions for the individuals involved in the hospital's/clinic's waste management system.
- Use of suitable collection and transport containers for clinical waste. For example, sharps (instruments such as cannulas, scalpels, etc.) may only be discarded in type-tested containers approved for this purpose.
c) Waste legislation
Waste legislation arose from the need to protect the health of the public and of people working in trade and industry. Today, waste legislation is part of the German Bundeskreislaufwirtschaftsgesetzes (KrWG), which is aimed towards protecting and conserving resources. Taking into account the increased goals for protecting the environment, waste legislation – for which the authorities of the individual Länder are responsible – determines how owners should deal with materials destined for disposal. The scope of public licensing, control and notification requirements is also based on a distinction between hazardous and non-hazardous waste.
Requirements for hospitals and clinics
With regard to hospital/clinic operators and/or the external companies contracted to manage waste, the Gewerbeabfallverordnung (GewAV) requires detailed records and documentation to be kept of the:
- Quantities of waste produced
- Composition of the waste
- Disposal pathways
These aspects especially concern the internal handing of waste and recyclable materials from medical devices. They are primarily relevant to the hospital/clinic in its capacity as waste producer. These aspects are reflected in:
- The separate collection of standard recyclable materials (glass, paper, light packaging, etc.)
- The legally compliant handling of healthcare-specific waste.
The responsibility for further “proper” handing of waste is transferred to a waste disposal company – certified for this type of waste – when the waste is handed over. Depending on the AS, proof of disposal may have to be provided for the final documentation.
Requirements for disposers and recyclers
The subsequent transport logistics, and shipment and treatment of the waste is regulated by transport legislation, the Abfallverbringungsgesetz and, lastly, by the KrWG again.
Tip
With regard to “waste for recovery,” legally the point during the disposal process at which it stops being considered waste and becomes a secondary raw material is legally important.
This dividing line is difficult to draw in the case of clinical waste containing valuable materials that have been in contact with patients, because of potential contamination or even infectivity. As already described, thermal treatment of the waste is sufficient to remove harmful substances. However, separate collection prior to such treatment is made difficult in practice by occupational health and safety regulations.
General requirements of the KrWG
The Kreislaufwirtschaftsgesetz (KrWG) was amended on October 20, 2020 to include three new paragraphs that offer producers of medical goods in particular more options for the value-preserving recycling of their devices after use:
§ 5 Legislative power to resolve legal difficulties
Article 5(2) establishes the authority to issue a statutory instrument on when something ceases to be waste much more extensively than before. A corresponding legal regulation could help solve the following problems:
Separately collected single-use surgical instruments made of chromium steel can be sent directly for metallurgical recycling. However, as long as these instruments are legally classified as waste, they cannot technically be put directly into a blast furnace, as blast furnaces are not authorized to incinerate waste.
The procedural steps required so that used instruments ultimately become scrap, i.e., a secondary raw material, remains a purely administrative question that still has to be clarified. Physically, of course, the material does not change when it ceases to be considered waste.
§ 9 Collection and treatment of waste
Article 9 of the KrWG address the separate collection and treatment of waste intended for recovery. This paragraph stresses that hazardous substances (including germs in the case of medical devices) must be removed as far as possible when the material is treated. The waste for recovery collected separately should (as far as possible) not just be used for energy recovery.
For valuable medical devices, in particular, this paragraph offers a good starting point for replacing the “recovery” that is currently carried out in waste incineration plants – across-the-board because it is cheap – with other processes in the future.
Therefore, an approach to economically attractive material that does not weaken public health protections requires:
- Valuable products to be separated before they end up in a hospital’s/clinic's general waste flow
- These recyclable materials to be combined to create a larger pool
§ 25 Take-back and take-back obligations
Article 25 establishes the basis for legally established requirements for take-back systems and take-back obligations, re-use, recovery and the disposal of waste generated after use of the products, up to and including contributions by device manufacturers to the costs of cleaning the environment.
This article reads as an implicit invitation to medical device manufacturers to proactively seek a suitable, preferably sector-wide, take-back system for their used goods before the legislature imposes one on them on its terms.
With regard to a contribution to clean-up costs, the discussion of the contamination of hospital wastewater by contrast media, among other things, is getting louder once again.
6. Opportunities for medical device manufacturers
a) New business models 1: pay-per-use & deposit return systems
New business models are enabling manufacturers to take steps towards a more sustainable future. For example, EP catheters contain platinum. Recyclers are very keen to buy these used disposable devices. That's because the precious metal is worth more than it would cost to dispose of the valueless remains.
A manufacturer of such instruments then has to buy platinum again on the market for their new instruments, then condition and shape it for use. If, instead, the manufacturer only leased its instruments to users for them to use, they would remain the property of the manufacturer and it could re-collect the instruments after use and re-use the platinum.
As a result, production and consumption models that, as well the economic use value, also attach importance to the preservation of the value of the material should be objectives. Deposit return schemes are one of the options for minimizing the return losses incurred with such pay-per-use models associated with material flows.
This means that the instruments are collected at the hospital/clinic and sent for the planned recovery with as little loss as possible. This also means that this strategy becomes more rewarding the higher the number of a manufacturer's products it is used for.
b) New business models 2: “clinic” recycling bin
As experience with take-back schemes for disposable surgical instruments has shown, the numbers of medical devices from a single manufacturer used (and the quantity of material in them) at individual hospitals and clinics are still rarely high enough for a take-back system to cover its costs.
As an alternative to proprietary individual solutions, a collection system that covers all recyclable materials, devices and materials (“clinic” recycling bin) could be set up. As the Institute for Recycling, Ecology & Design has found, compliance with special occupational health and safety regulations is not a cost-driving factor unless infectiously contaminated waste is involved.
A sector-specific, cross-manufacturer take-back platform also offers numerous options in terms of waste legislation:
Provided that the waste is non-hazardous commercial waste, there is no obligation to transfer waste (according to Article 17 KrWG) or notify the waste management authorities. A voluntary take-back scheme (§26 KrWG) for the manufacturer's own products could be set up via the recyclable materials platform – however, this would require an official assessment notice. Alternatively, the collected recyclable materials can be classified as a) waste for recovery or b) electronic waste (according to AVV)
Provided that both categories concern non-hazardous waste, no administrative authorization would be required. The actual disposal is carried out by certified specialist companies in both cases.
c) Competitive advantages through cooperation between manufacturers
Hospitals and clinics and their group purchasing organizations don’t want to become dependent on a single manufacturer. If selected manufacturers joined forces when it came to recovery as well, this would have advantages at various levels:
- This group of manufactures would have a competitive advantage, market power and the chance to differentiate itself from other manufacturers, e.g., those from low-wage countries.
- Working together, they would be able to comprehensively collect and preserve recyclable materials.
- The costs of collection and logistics would be shared.
- The quantity and mix of materials would be more economically attractive for companies that recover materials.
- The waste producers (hospitals/clinics) do not need to put as much effort into separating the waste because it does not need to be separated according to manufacturer.
- Recycling reduces the amount of waste to be disposed of by hospitals and clinics and, therefore, the costs of doing so.
- A joint initiative makes it easier to comply with the documentary requirements established by the GewAV with regard to waste quantities, waste types and the destination of the waste.
- The new business models outlined above would still be options for manufacturers.
But a joint initiative would also benefit hospitals and clinics:
- It would simplify disposal (process & documentation)
- It would mean no pre-sorting is necessary
- There would be reduced need for space for separate collection containers
- Reduction of disposal costs (volume reduction or discounts)
- Lower purchasing costs through pay-per-use models
d) Practicing communicable sustainability
Efforts to implement sustainable recycling shouldn’t just be driven by cost and profit considerations. It should also be about improved sustainability:
- We should pollute the environment with less trash
- We should be more careful with the resources available to us (energy, raw materials)
Nevertheless, sustainable business practices can be effectively communicated by both manufacturers and operators as part of their public image.
7. Requirements for effective recycling
a) General willingness to innovate
Medical devices generally have to undergo complex, lengthy and, therefore, expensive testing procedures before they can be authorized. The MDR has even raised these hurdles with the aim of ensuring patients are better protected.
However, the sustainable use of resources is not one of the test criteria for medical devices established by the legislation. The idea of an authorized device being changed just to improve the recyclability of its materials will remain wishful thinking until the legislator makes it recyclability a requirement.
The fish rots from the head: it is and remains the task of senior management to make recycling and sustainability company goals and to ensure that these goals are met.
b) Recycling-friendly device design
If functional or design changes to a device require a new round of testing to be carried out, manufacturers have an opportunity to redesign their devices taking sustainability aspects into account. For example, when re-engineering a high-quality disposable endoscope, it should be ensured that
- Fewer different materials are used
- The materials used can be separated from each other easily
- The materials selected are attractive in terms of recovery
- Reprocessed parts can be re-used in a device if necessary
c) Support from policy makers and technical bodies
As explained above, there is a conflict of objectives between
- the primacy of hygiene, i.e., the requirements for substantial occupational health and safety, public health protection and, in some cases, additional infection control and
- the primacy the KrWG gives to material recovery.
This conflict of objectives can be explained by the historical development of the two sets of regulations and a practical balance is required in order to give greater weight to sustainability in the case of medical devices.
Unfortunately, the bodies legally responsible for authorizations do not use the room for interpretation to encourage resource conservation due to an excess of caution. They feel a stronger commitment to the current regulations on occupational health and safety and public health protection.
For these bodies to be able to make decisions with a greater degree of legal certainty, including decisions regarding sustainability, clearer definitions are required regarding, for example, the processes and the locations (production sites) where waste becomes raw material.
In the future, further coordination between waste, occupational health and safety and public health regulations will be necessary to ensure circular economy processes are given regulatory encouragement as well. This requires flanking support from politics and the constructive participation of regulatory and professional trade associations (#RegulatoryScience).
8. Summary and conclusion
a) Status quo
Sustainable management of the materials used in medical devices is often still more expensive than the current linear manufacturing model:
- Mine raw materials
- Produce in low-wage countries
- Sell at a high price in Europe
- Use devices and then throw them away
- Dispose of devices, mostly through incineration
Hospitals and clinics are under significant cost pressures. As a result, they are only willing to engage with sustainability issues to the extent required by law. In contrast, proposals that help keep operating costs down in the short and medium term will be implemented.
It is true that waste disposal is a significant factor on the balance sheet. But, the particularly high-value medical devices mentioned above currently only account for a negligible proportion in terms of volume and mass (with the exception of large equipment and complete operating theater facilities).
The legal situation exacerbates the tendency to incinerate devices rather than recycle them if there is any doubt.
With rising costs for raw materials and for disposal, and with increasing environmental awareness, this approach is reaching its limits.
b) Steps towards greater sustainability
Manufacturers and hospitals/clinics would be well advised to sustainably increase the proportion of materials from medical devices that they recycle, for economic as well as environment reasons.
New joint strategies for recovering recyclable materials
- Identify potential project partners. Cooperate with the market leaders in the fields of clinical supply, clinic operation, logistics & waste disposal.
- Together work out the specific “clinical” waste/potential recyclables within the framework of the existing disposal and recovery structures.
- Work together to develop cost-effective disposal strategies.
- Set up a logistically sustainable collection system (“clinic” recycling bin) that covers all recyclable materials, devices and manufacturers and complies with the occupational health and safety regulations.
- Set up a pilot project for sorting waste and potential recyclables from hospitals and clinics, healthcare operators, veterinary facilities and forensic medical facilities.
- Classify the used materials in terms of their re-use or recycling and reprocessing.
Take advantage of scientific support when taking these steps. This also applies to the development and technical adaptation of innovative separation, sorting and classification processes geared towards specific recyclable materials.
Recyclability “by design”
Adapt your products and manufacturing processes for the production of MDR-compliant medical devices so that they re-use or even avoid using raw materials.
Recycling and preserving resources should be a concern for everyone and all organizations. Talking about sustainability is not enough. It’s time for action.
The Institute for Recycling, Ecology and Design (IRED) in Frankfurt am Main and its research partner, the Fraunhofer Research Institution for Materials Recycling and Resource Strategies (IWKS) in Alzenau, help manufacturers of medical devices, recycling companies and hospitals and clinics in all phases of the implementation of the actions described above through their combined scientific expertise and recycling network.
For more information, please contact Prof. Dipl.-Phys. Werner Lorke (lorke(at)ired-institute.com) from the Institute for Recycling, Ecology and Design (IRED).