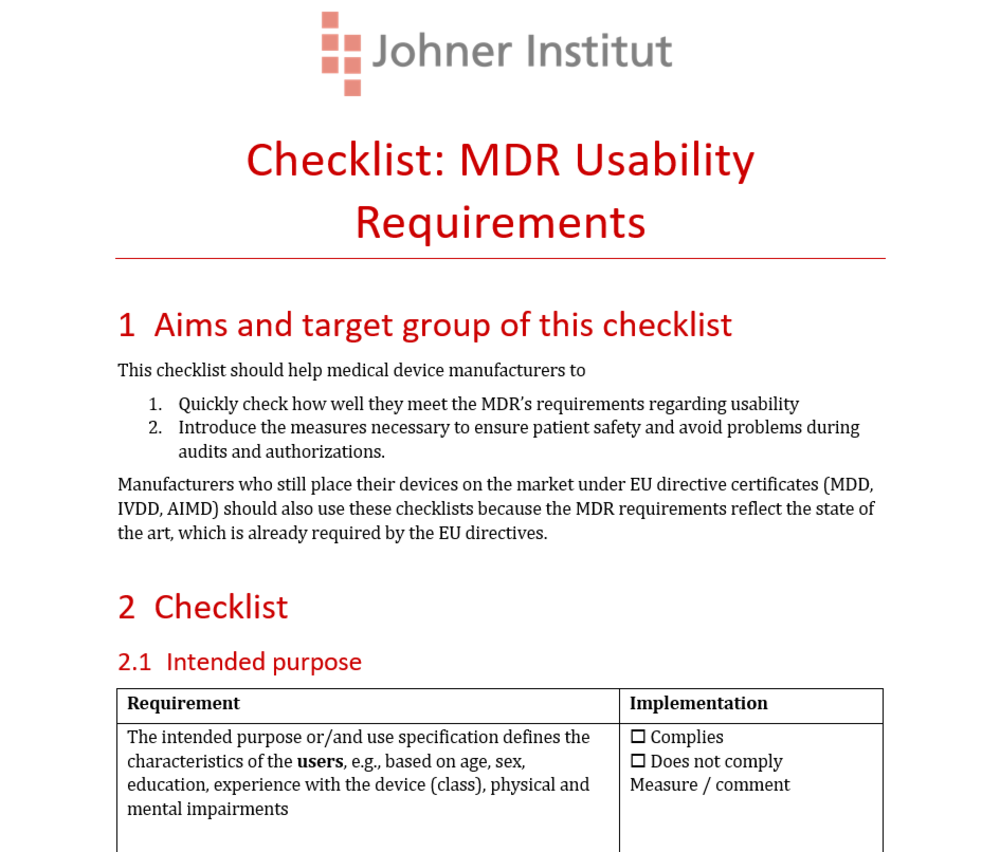
The MDR's Usability / Human Factors Requirements
Manufacturers must demonstrate compliance with the MDR's usability requirements for all medical devices without exception.
For some devices, there are transitional periods. Nevertheless, manufacturers would be well advised to familiarize themselves with the differences between the MDD's and the MDR's requirements with regard to usability. That's the only way to manage the transition to the MDR smoothly and avoid regulatory hassle and costs, e.g., from unnecessary usability tests.
Our free checklist will help you to quickly make sense of it all and identify the measures necessary, even for “legacy devices”.
1. A look back: the MDD's usability requirements
The original version of the Medical Device Directive (MDD, 93/42/EEC) already contained some initial usability requirements. For example, “measurement, monitoring and display scale[s]” had to be “designed in line with ergonomic principles”.
The amending directive 2007/47/EC added to the MDD the explicit essential requirement that risks caused by a lack of usability must be minimized. It already differentiated between risks arising from the
- “ergonomic features of the device”
- “environment in which the device is intended to be used”
- “technical knowledge, experience [...] and training and where applicable the medical and physical conditions of intended users”
In parallel with this amendment, the EU harmonized the standard EN IEC 62366:2006, which, from then on, represented the state of the art.
2. The MDR's usability requirements in detail
Searching the EU Medical Device Regulation (MDR) for the term “usability” returns surprisingly few hits. Nevertheless, the EU regulation contains numerous usability requirements.
a) Article 5 and Annex I, paragraph 1: devices must be suitable for their intended purpose
Article 5 and the first paragraph of Annex I (General safety and performance requirements) of the MDR establish very similar requirements:
Ein Produkt muss unter Berücksichtigung seiner Zweckbestimmung den in Anhang I festgelegten für das Produkt geltenden grundlegenden Sicherheits- und Leistungsanforderungen genügen.
Die Produkte erzielen die von ihrem Hersteller vorgesehene Leistung und werden so ausgelegt und hergestellt, dass sie sich unter normalen Verwendungsbedingungen für ihre Zweckbestimmung eignen.
Both refer to the intended purpose. This intended purpose should not just define the intended medical purpose, it should also define the intended users and the environment in which the device is intended to be used. IEC 62366-1 calls this the “use specification”.
Additional information
Read more on what an intended purpose should include here.
This means that manufacturers who have not precisely defined this intended purpose and, therefore, have not defined the intended users and the environment in which the device is intended to be used will find it very difficult to comply with the MDR's usability requirements.
The first paragraph of Annex I makes providing this proof mandatory.
b) Annex I, paragraph 3: analysis of foreseeable misuse
The third paragraph of Annex I on general safety and performance requirements contains even more specific usability requirements:
It requires manufacturers to have a risk management system. They must use it to “estimate and evaluate the risks associated with, and occurring during, the intended use and duringreasonably foreseeable misuse”.
This risk analysis must correspond to the state of the art. Pure speculation is, therefore, not enough. In fact, the following would be expected:
- Careful research and analysis of foreseeable misuse of comparable devices, e.g., in regulatory databases and the manufacturer’s own post-market data
- List of alluse scenarios with subsequent task analysis
- Monitoring of users with authorized devices or as part of formative and summative evaluations
An auditor or a regulatory authority would expect the results of this analysis to be in the risk management file.
c) Annex I, paragraph 5: measures to eliminate or reduce risks related to use errors
The authors of the MDR have copied paragraph 5 from the MDD almost to the letter:
Beim Ausschluss oder bei der Verringerung der durch Anwendungsfehler bedingten Risiken müssen die Hersteller
a) die Risiken aufgrund ergonomischer Merkmale des Produkts und der Umgebung, in der das Produkt verwendet werden soll, so weit wie möglich verringern (auf die Sicherheit des Patienten ausgerichtete Produktauslegung) sowie
b) die technischen Kenntnisse, die Erfahrung, die Aus- und Weiterbildung, gegebenenfalls die Anwendungsumgebung sowie die gesundheitliche und körperliche Verfassung der vorgesehenen Anwender berücksichtigen (auf Laien, Fachleute, Behinderte oder sonstige Anwender ausgerichtete Produktauslegung).
MDR, Annex I, paragraph 5
While paragraph 3 required the analysis of usability risks, paragraph 5 calls for their elimination, or reduction at least.
As a result, the MDR makes it mandatory for manufacturers to define measures for all identified risks related to usability. According to paragraph 4, manufacturers must first aim for inherent safety, then aim for protection measures and, only after that, provide information for safety.
Examples of these measures are:
- Inherent safety: a switch that does not exist cannot be used incorrectly.
- Protection measure: a switch that has a flap to prevent it being pressed accidentally.
- Information for safety: a warning, e.g., in the instructions for use, regarding the consequences of pressing the switch by mistake.
d) Annex I, paragraph 14.1: specific risks from use in combination with other devices
Paragraph 14 requires risks resulting from the interaction of devices with their environment to be controlled. These risks are also, in part, related to usability:
Wenn ein Produkt zur Verwendung in Kombination mit anderen Produkten oder Ausrüstungen bestimmt ist, muss die Kombination einschließlich der Verbindungen sicher sein und darf die vorgesehene Leistung der Produkte nicht beeinträchtigen. Jede Einschränkung der Anwendung im Zusammenhang mit solchen Kombinationen wird auf der Kennzeichnung und/oder in der Gebrauchsanweisung angegeben. Vom Anwender zu bedienende Verbindungen, wie etwa die Übertragung von Flüssigkeit oder Gas oder elektrische oder mechanische Verbindungen, werden so ausgelegt und hergestellt, dass alle möglichen Risiken, wie etwa fehlerhafte Verbindungen, so gering wie möglich gehalten werden.
MDR, Annex I, paragraph 14.1
These usability requirements in the MDR should already have been met if the manufacturer has considered all use scenarios, including scenarios in which their device is combined with and connected to other devices. The MDR also demands here, as of a measure, inherent safety against faulty connections as far as possible.
e) Annex I, paragraph 14.2: specific risks resulting from inadequate ergonomic features
Paragraph 14.2 looks at another type of risk. Unlike paragraph 14.1, it requires risks relating to the design of the device to be controlled not risks resulting from misuse. The MDR refers to “ergonomic features”:
Die Produkte werden so ausgelegt und hergestellt, dass folgende Risiken ausgeschlossen oder so weit wie möglich reduziert werden:
a) Verletzungsrisiken im Zusammenhang mit den physikalischen Eigenschaften einschließlich des Verhältnisses Volumen/Druck, der Abmessungen und gegebenenfalls der ergonomischen Merkmale des Produkts;
MDR, Annex I, paragraph 14.2
An example would be a dialysis machine on wheels. Due to its weight, a rolling dialysis machine would be difficult to stop and could cause bruising to someone's feet or hands. This is exactly the type of risk that manufacturers must minimize.
f) Annex I, paragraph 14.6: ergonomics of displays
Paragraph 14.6 of the MDR also establishes usability requirements.
Mess-, Kontroll- oder Anzeigeeinrichtungen werden so ausgelegt und hergestellt, dass sie mit Blick auf die Zweckbestimmung, die vorgesehenen Anwender und die Umgebungsbedingungen, unter denen die Produkte verwendet werden sollen, ergonomischen Grundsätzen entsprechen.
MDR, Annex I, paragraph 14.6
But the MDR does not explain what ergonomic principles actually are. As a result, a lot of manufacturers are starting to define their own style guides and, to a large extent, reinvent the wheel over and over again.
Tip
The Johner Institute recommends using the specifications found in the ISO 9241 family of standards. These standards describe, in concrete terms, how user interfaces - from menus to web pages to command lines - should be designed.
Please note: The effects on the design of user interfaces must take the use environment (e.g., brightness, distance from the device) as well as the users (e.g., physical impairments) into account.
g) Annex I, paragraph 21.3: Understandability of displays
In addition to ergonomic requirements, the MDR also requires displays to be understandable:
Die Funktion von Bedienungs- und Anzeigeeinrichtungen wird auf den Produkten deutlich angegeben. Sind die Anweisungen für die Anwendung des Produkts auf diesem selbst angebracht oder werden die Betriebs- oder Regelungsparameter visuell angezeigt, so müssen diese Angaben für den Anwender und gegebenenfalls den Patienten verständlich sein.
MDR, Annex I, paragraph 23.1
In order to meet this MDR usability requirement, manufacturers must define the characteristics of the users and the patients precisely. Because whether a display is understandable or not depends on the prior knowledge and experience of the specific user.
Whether these displays are really understandable as part of the user interface can only be proven by empirical data. Manufacturers can collect this data through, for example, surveys, participant observations or post-market data.
h) Annex I, paragraph 22: lay persons
The specific and extensive requirements contained in the MDR regarding safe use by lay persons are new in this form. The regulation even defines the term:
Definition: Lay person
“‘lay person’ means an individual who does not have formal education in a relevant field of healthcare or medical discipline;”
Because the requirements are so extensive, here is a summary:
- Manufacturers must take the “variation that can be reasonably anticipated in the lay person's technique and environment” into account.
- In any case, the risks must be minimized. The lay person must be able to use the device “safely and accurately [...] at all stages of the procedure”.
- This may require “appropriate training and/or information”.
- Instructions and information must be “easy for the lay person to understand and apply”.
- As a quasi-risk-minimizing measure, the MDR stipulates that the lay person themselves should be able to verify that the device “will perform as intended by the manufacturer” and that the device should emit a warning if it has “failed to provide a valid result”.
These requirements have a direct effect on the design of the device, as well as on the design of training courses and the evidence required, e.g., in the form of usability tests.
i) Annex I, paragraph 23: usable instructions for use
The MDR does not limit its requirements to the device itself. The instructions for use and other accompanying materials must also be usable.
Medium, Format, Inhalt, Lesbarkeit und Anbringungsstelle der Kennzeichnung und der Gebrauchsanweisung eignen sich für das jeweilige Produkt, seine Zweckbestimmung und die technischen Kenntnisse, die Erfahrung, Ausbildung oder Schulung der vorgesehenen Anwender. Insbesondere ist die Gebrauchsanweisung so zu verfassen, dass sie von dem vorgesehenen Anwender ohne Schwierigkeiten verstanden wird, und gegebenenfalls mit Zeichnungen und Schaubildern zu ergänzen.
MDR, Annex I, paragraph 23.1 c)
Manufacturers are not entirely free to implement these requirements as they see fit. For example, the MDR requires them to use internationally recognized symbols and to describe them where necessary.
j) Annex II: technical documentation
Even Annex II contains a direct reference to usability because it establishes the contents of the technical documentation. This should include:
- A definition of the intended users
- A description of which other devices the device can/should be combined with/connected to
- Tests and test results
For software, these tests must be performed in a “simulated or actual user environment”.
If the device is intended to be connected to another device, “proof that it conforms to the general safety and performance requirements when connected to any such device(s) having regard to the characteristics specified by the manufacturer” must be provided.
k) Article 83(3) and Annex III section 1.1: post-market surveillance
The only time that the MDR uses the term “usability” is in the context of the UDI (not relevant here) and the post-market surveillance.
The MDR expects manufacturers to use “data gathered by the manufacturer's post-market surveillance system” in particular:
f) Ermittlung von Möglichkeiten zur Verbesserung der Gebrauchstauglichkeit, der Leistung und der Sicherheit des Produkts;
MDR, Article 83(3)
In Annex III, the MDR then specifies which information has to be collected and analyzed:
– Informationen über schwerwiegende Vorkommnisse, einschließlich Informationen aus den Sicherheitsberichten, und Sicherheitskorrekturmaßnahmen im Feld,
– Aufzeichnungen über nicht schwerwiegende Vorkommnisse und Daten zu etwaigen unerwünschten Nebenwirkungen,
– Informationen über die Meldung von Trends,
– einschlägige Fachliteratur oder technische Literatur, Datenbanken und/oder Register,
– von Anwendern, Händlern und Importeuren übermittelte Informationen, einschließlich Rückmeldungen und Beschwerden und
– öffentlich zugängliche Informationen über ähnliche Medizinprodukte.
For manufacturers this means:
- Checking that all the required information is collected and analyzed. This must also be described in the corresponding process instruction.
- Ensuring that this information is also analyzed for indications of how to improve the usability. Ideally, manufacturers will be able to demonstrate this with examples.
- Ensuring that usability experts and risk managers are involved in this process.
3. What specifically should manufacturers do?
a) Manufacturers of new devices
Manufacturers developing new devices should follow IEC 62366-1. This standard describes the state of the art.
b) Manufacturers of “legacy devices”
In contrast, manufacturers of devices that have already been developed and that either have to be re-authorized or that benefit from transitional periods should evaluate the conformity of their devices again.
This does not necessarily require a complex conformity assessment. The Johner Institute’s checklist (see below) will help quickly provide some initial clarity.
c) All manufacturers
Systematically collecting post-market data and analyzing it for indications on how to improve usability is an unavoidable obligation. This requires suitable processes, tools and experts.
d) Summary
In summary, the Johner Institute makes the following recommendations:
- For devices placed on the market for the first time under the MDR, follow IEC 62366-1 strictly
- For all other devices, work through the checklist referred to below, identify gaps and implement corresponding measures to close those gaps. Use external support if resources are scarce
- Establish an effective post-market surveillance system for all devices
N.B!
Careful! Manufacturers of devices that benefiting from the transitional periods should not sit back and relax on the assumption that conformity with the MDD is a given and sufficiently proven. The state of the art and risk management forces manufacturers to de facto comply with the MDR requirements, even though the MDD never worded these requirements as explicitly.
4. Checklist and further support from the Johner Institute
a) Checklist
The Johner Institute has prepared a checklist that manufacturers can use to give themselves a quick overview of whether their devices and processes comply with the state of the art and the MDR's requirements.
b) Identifying gaps and establishing measures
The Johner Institute's usability experts will help you to quickly identify deviations and to establish the perfect measures for closing these gaps.
Tasks that the Johner Institute can support you in are:
- Review of existing usability files for conformity with IEC 62366-1
- Checking that the risk management files are complete and conform to the MDR and ISO 14971
- Analysis of process descriptions, e.g., for post-market surveillance
- Review of the instructions for use to check they are understandable and comply with the relevant laws and standards
c) Implementing measures as quickly and as easily as possible
The Johner Institute's experts focus on avoiding any type of unnecessary work and work on a risk-based basis. They work with you towards the aim of ensuring safety for patients and confidence for your company during when it comes to authorization audits.
Tasks that the Johner Institute can support you in are:
- Revising and honing the use specification (to avoid unnecessary usability tests)
- Describing any missing use scenarios and assessing their relevance for safety.
- Suggesting improvements to the user interface
- Amending (or creating) instructions for use to ensure they conform to the MDR
- Performing formative and summative evaluations
- Defining your post-market process and supporting you with the analysis of your data
The Johner Institute has already performed post-market surveillance for some manufacturers as part of a beta program. This service will soon be offered to other manufacturers.
5. Conclusion and summary
A first look at the MDR is deceptive. You shouldn’t conclude that usability is not a concern for the MDR just because the EU regulation barely uses the term “usability”. In fact, the opposite is true. As explained in the article above, it establishes extensive usability requirements.
Unfortunately, the MDR uses terms like “ergonomics” (like the German Digital Healthcare Applications Ordinance (DiGAV)), but does not use established definitions.
Manufacturers of devices that are still placed on the market with MDD certificates should not sit back and relax either. The MDD also demands the state of the art. The MDR just defines this state of the art more explicitly.
The Johner Institute recommends that manufacturers should
- Get a quick overview using the free checklist.
Make sure that their post-market surveillance system is effective and conforms to the requirements. Because there are no(!) transitional periods for this.