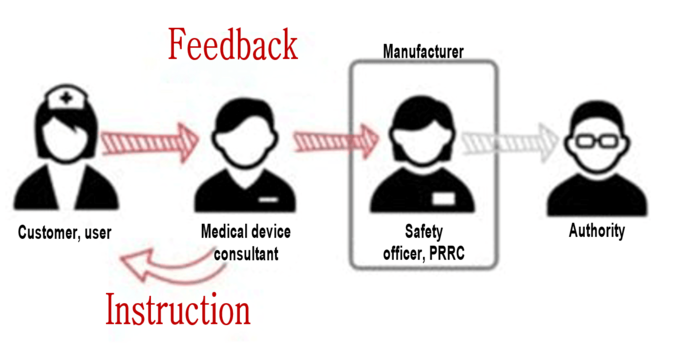
Medical Device Consultant – Only A German Concept?
Medical device consultants play a key role in guaranteeing the safety of medical devices. However, only German medical device law (MPG, MPDG) requires the role of “medical device consultant.” Neither the EU directives (MDD, IVDD, AIMDD) nor the EU regulations (MDR, IVDR) include this concept.
Manufacturers should not only be aware of the changes made to the role by the MPDG, they should also be aware of all regulatory requirements related to the role of medical device consultant. And they’re not just found in German laws.
1. People who count as medical device consultants
a) Definitions
A medical device consultant is – according to the MPG/MPDG – any person who provides technical information to experts or instructs them in the proper handling of medical devices on a professional basis.
The MPG and MPDG also define the term experts:
Definition: Experts
“Members of the health professions, the health industry or institutions active in the field of health as well as other persons who manufacture or test medical devices or, in the exercise of their profession, place medical devices on the market, implant, put into service, operate or use them.”
Source: MPG and MPDG
Standard examples of experts include:
- Doctors
- Nursing staff
- Medical technical assistants
- Staff working in medical technology, laboratories and, where applicable, hospital IT
- Service technicians
Patients are not included in this list.
b) Typical roles and occupations
Medical device consultants are typically people who work in the field or application consultants, sometimes also known as “application engineers".
For example, medical device consultants show dialysis specialists how to prepare a dialysis machine for a treatment and how to clean it afterwards. Medical device consultants are present in the operating theater and instruct surgeons on the use of heart-lung machines and the implantation of hip joints.
If people who work in a manufacturer’s office or call center also provide advice or instruction, they also fall under this definition.
Office tasks that are not part of the role of the MDC | Office tasks that are part of the role of the MDC |
A customer calls the office or call center and asks for a product specification that the employee can read off the data sheet. For example, the employee asked about a dental drill's revolutions per minute. | A customer calls the office or call center and asks how to use a device or whether it is suitable for a specific indication. For example, the employee is asked whether the drill can also be used to fixate a particular implant. |
2. Activities of medical device consultants
Medical device consultants instruct and inform users (experts) on the use of medical devices. At the same time, medical device consultants are under the obligation to report problems, risks and side effects that have occurred with these devices to the manufacturer.
a) Instruction
It is usually the manufacturer who decides whether instruction on the use of a medical device is necessary. However, this instruction is mandatory for some medical devices. The Medizinproduktebetreiberverordnung states:
“The operator may only operate a medical device listed in Annex 1 if the manufacturer or person authorized by and acting with the agreement of the manufacturer:
1. has subjected this medical device to a functional test at the place of operation and
2. has instructed the person authorized by the operator in the correct handling, use and operation of the medical device and in the permissible combinations with other medical devices, objects and accessories on the basis of the instructions for use and the accompanying safety information and maintenance instructions.”
Section 10 Medizinproduktebetreiberverordnung
This Annex 1 lists critical medical devices, such as MRI devices, defibrillators, perfusors and electrosurgical knives. These medical devices are also referred to as being subject to instruction.
For these devices, the legislator requires not only that a representative of the operator, e.g., the hospital, receives instruction, as described above, but also that all users of the devices receive instruction – typically from the medical device consultant.
This instruction is often given on the device directly, as already described above with the examples of the dialysis machine and the implants.
NB!
Careful: Section 5 of the MPBetreibV explicitly requires: “(3) The […] instruction of the person authorized by the operator according to paragraph 1 no. 2 must be documented.”
b) Training
This instruction is regularly accompanied by training. Such training should address the following topics:
Topic | Example: dialysis machine |
Medical foundations | Kidneys, hormone system |
Therapeutic possibilities | Dialysis procedures, transplantation |
Device type and specific device | Hemodialysis machines, accessories, instructions for use, symbols, if applicable safety and metrological controls |
Use | Preparation, adjustment, cleaning, troubleshooting |
Notification requirements | Contact details of the manufacturer, medical device consultant and BfArM, reporting deadlines and forms |
c) Feedback
Medical device consultants are also under the obligation to report problems back to the manufacturer.
“(4) The medical devices consultant shall take written record of reports from experts bearing on adverse effects, interactions, malfunctions, technical defects, contra-indications, falsifications or other risks associated with medical devices and transmit themin writing or electronically without delay [...].”
MPG Section 31 and MPDG Section 83
The law lists several types of “problems”:
“Problem” | Example |
Side effect | Patient has an allergic reaction to contact with the medical device |
Interaction | Cell phone interferes with the display of a medical device |
Malfunction | Mechanical jamming, breaking of a component, system displays data of the wrong patient |
Technical defect | Protective film peels off |
Contraindications | Metal implant causes problems in MRI scans |
So, the law specifies:
- What has to be report: see above
- Who it has to be reported to: this is the manufacturer (safety officer or person responsible for regulatory compliance) or the importer. In this case, the MPG and MPDG differ (see below)
- The deadline for the report: immediately, i.e., usually the same day
- The form to use for the report These requirements are described in the MDR/IVDR and the MPDG and the Medizinproduktesicherheitsplanverordnung (MPSV).
Tip
As a medical device consultant, always ask for acknowledgment of receipt of your report!
Medical device consultants are thus an essential part of the manufacturer's post-market surveillance system (see Fig. 2).
3. Requirements for a medical device consultant’s expertise
The MPG and MPDG establish almost identical demands for the expertise a medical device consultant must have.
The medical device consultants themselves must always be trained in the medical devices they provide instruction for. In addition, they need either relevant training or skills acquired through, generally, one year of practical experience.
NB!
Medical device consultant training must be at least partially device-specific and must also be continuously updated. This means that there can be no ‘one-size-fits-all’ training for medical device consultants. However, the basic principles, particularly the legal principles, can be taught in a non-device-specific way, e.g., by watching the training videos in Auditgarant.
4. Regulatory requirements
a) Comparison of the requirements of the MPG and the MPDG
The Medizinproduktedurchführungsgesetz (MPDG) keeps the requirement for a medical device consultant and only makes minor changes to the equivalent article in the Medizinproduktegesetz (MPG). The changes are highlighted in bold in the table below.
| MPG Section 31 | MPDG Section 83 |
Required expert knowledge | (1) Any person who provides technical information to experts or instructs them in the proper handling of medical devices on a professional basis (medical devices consultant), may only carry out such an activity if he/she possess, for the specific medical device, the requisite expert knowledge as well as the experience needed to provide information and, where necessary, instruction in the handling of specific medical devices. The first sentence shall also apply to information given by telephone. | 1) Any person who provides technical information to experts or instructs them in the proper handling of medical devices on a professional basis (medical devices consultant), may only carry out such an activity if he/she possess, for the specific medical device, the requisite expert knowledge as well as the experience needed to provide information and, where necessary, instruction in the handling of specific medical devices. The first sentence shall also apply to information given by telephone. |
Definition of expert knowledge | (2) The following shall be deemed to have the necessary expert knowledge: 1. any person who has successfully completed a course of studies in a natural science, medical or a technical profession and has received training on specific medical devices, or 2. any person who, for a period of at least one year, in justified cases also for a shorter period of time, has gained the necessary experience in informing and, where necessary, instructing others in the handling of specific medical devices. | (2) The following shall be deemed to have the necessary expert knowledge: 1. any person who has successfully completed a course of studies in a natural science, medical, technical or commercial IT profession and has received training on specific medical devices, or 2. any person who, for a period of at least one year, in justified cases also for a shorter period of time, has gained the necessary experience in informing and, where necessary, instructing others in the handling of specific medical devices. |
Proof of expert knowledge | (3) The medical devices consultant shall furnish the competent authority, upon request, with proof of his/her expert knowledge. The medical devices consultant shall have up-to-date knowledge of the specific medical devices so as to be able to provide competent advice. The sponsor shall ensure that the medical devices consultant receives regular training. | (3) The medical devices consultant shall furnish the competent authority, upon request, with proof of his/her expert knowledge. The medical devices consultant shall have up-to-date knowledge of the specific medical devices so as to be able to provide competent advice. The sponsor shall ensure that the medical devices consultant receives regular training. |
Feedback obligation | (4) The medical devices consultant shall take written record of reports from experts bearing on adverse effects, interactions, malfunctions, technical defects, contra-indications, falsifications or other risks associated with medical devices and transmit them in writing or electronically without delay to the person responsible according to Section 5, sentences 1 and 2 or that person's safety officer for medical devices. | (4) The medical devices consultant shall take written record of reports from experts bearing on adverse effects, interactions, malfunctions, technical defects, contra-indications, or other risks associated with medical devices and transmit them in writing or electronically without delay to the manufacturer, its authorized representatives or their person responsible for regulatory compliance. If medical devices are placed on the market under the responsibility of the importer, the information pursuant to sentence 1 must be transmitted in writing or electronically. |
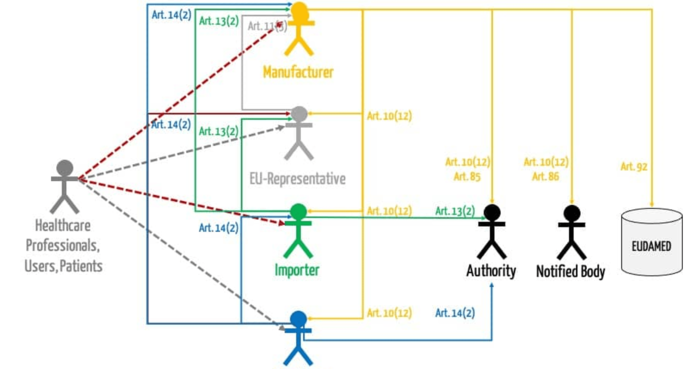
b) MDR requirements
The MDR and IVDR do not include the concept of medical device consultants. However, both EU regulations establish extensive post-market surveillance requirements.
Additional information
Read more about what you need to know to establish an MDR-compliant post-market surveillance system here.
c) ISO 13485
ISO 13485 does not require manufacturers to qualify and use medical device consultants either. It does, however, require manufacturers to determine the regulatory requirements that apply to them. These include the MPG and the MPDG.
In addition, ISO 13485 requires human resources to be made available and their competence to be guaranteed.
The standard also requires a procedure for the feedback process to be established. Outsourced processes must be monitored by manufacturers.
5. FAQs on medical device consultants
a) What requirements are there for people who train medical device consultants?
The laws do not set any requirements for the qualifications of the trainers. They don’t even specify how medical device consultants should be trained.
The Johner Institute recommends:
- Providing regulatory training, e.g., through online training, and checking the effectiveness of the training. Auditgarant is one way of doing this.
- The concepts and the documents used by the medical device consultant to train experts should be reviewed by the device manager, the risk manager, the person responsible for regulatory compliance and the quality manager.
- Documented trial training within this group will help you answer potential questions from authorities.
- Medical device consultants who have not received instruction or training for a long time should be given refresher courses.
- The regulatory affairs department should determine whether medical device consultants need to be given new training when laws are changed.
- In the event of modifications to medical devices and new medical devices, the (new) medical device consultants must be re-trained. This should be part of the release process.
b) What should I do as a manufacturer if the medical device consultants are employed by, e.g., a dealer rather than me?
In terms of the MPG and the MPDG, it does not matter whether the people who instruct and train experts on a professional basis are employed by a manufacturer, a dealer or another company, or are freelancers. They all have to follow these laws.
As a manufacturer you are obliged:
- To establish and maintain a functioning post-market surveillance/feedback system.
- To minimize risks resulting from incorrect or insufficient training.
Therefore, regardless of the requirements that medical device consultants must meet, you must ensure through contracts that only trained people instruct experts and that they reliably report all problems back to you as the manufacturer immediately.
c) The law regulates what the MDCs have to do. Does it also say what they must NOT do?
The MPG and the MPDG do not set out what the medical device consultants are not allowed to do. However, it follows from other laws that medical device consultants can only participate in an examination or treatment with the consent of the patient and, of course, must not perform them themselves.
They must not touch patients or even participate in surgical procedures.
6. Conclusion, summary
The concept of medical device consultant is a German one. But comparable requirements can be found in the MDR and IVDR as well as other European countries:
- Risks from medical devices must be minimized. The correct and complete instruction of users is an important element of risk minimization.
- The competence of the people giving this instruction must be guaranteed and documented.
- For patient safety, it is essential that problems with medical devices (unknown side effects, malfunctions, technical defects, etc.) are immediately reported to the manufacturer or importer. It is also the responsibility of these manufacturers and importers to ensure that this feedback is actually provided.
Up to now, authorities and notified bodies have been very reluctant to check whether the people who the law defines as medical device consultants are actually complying with the regulatory requirements. This is likely to change in the future with the stricter requirements for post-market surveillance.
The Johner Institute offers public seminars and in-house training for Medical Devices Consultants.
Here you will find information on the next dates and how to register. If nothing to the contrary is noted in the table on the overview page.
Currently our registration process is still in German, so in case of any questions or difficulties please contact our Seminar team directly.
If you do not find this seminar in the calender, it is always available as inhouse-seminar. Please contact our Seminar team directly.