ISO/IEC/IEEE 15289: Clarity at Last for Software Documentation?
The title of ISO/IEC/IEEE 15289 is “Systems and software engineering — Content of life-cycle information items (documentation)”.
As the title suggests, it establishes specifications for the content of software documentation. This makes the standard ideal for:
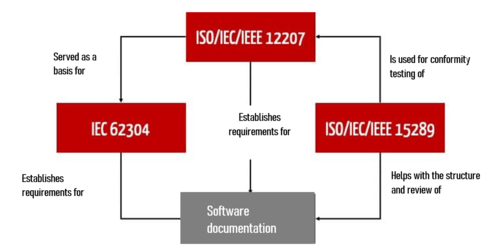
- Helping with the implementation of other standards, such as ISO/IEC/IEEE 12207:2017 and IEC 62304
- Checking conformity with these standards
- Identifying the documents required for the software documentation
- Defining the contents of these documents
This means that ISO/IEC/IEEE 15289:2019 is a standard that affects every organization that develops software.
1. Overview of ISO/IEC/IEEE 15289
a) Target group: who should read the standard?
The standard is aimed at people involved in software documentation in the following roles:
Role | Responsibilities in the context of ISO/IEC/IEEE 15289 |
Project manager | Defining the information management process Determining the documentation requirements (and therefore the documents and their content) |
Buyer (of the software) | Defining the requirements for the documentation that the manufacturer must produce |
Developer (e.g., architecture, implementation, testing) | Writing the software and systems development documentation |
Quality manager | Checking conformity with standards (ISO/IEC/IEEE 12207 and ISO/IEC/IEEE 15288 are mentioned in this regard) |
b) Section structure of ISO/IEC/IEEE 15289
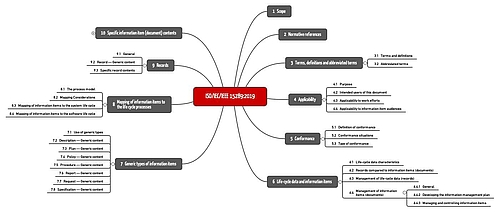
ISO/IEC/IEEE 15289:2019 contains 10 sections across 86 pages.
You can download the mind map as a PDF here:
ISO-IEC-IEEE-15289-2019Download
The following table gives an overview of the individual sections of the standard.
Section | Title | Summary |
1 | Scope | The standard wants to describe the content of all documents in a software documentation but not a management system |
2 | Normative references | References to ISO/IEC/IEEE 12207 and ISO/IEC/IEEE 15288 |
3 | Terms, definitions and abbreviated terms | 29 definitions. But the document classes are only defined for the first time in section 7 |
4 | Applicability | Purpose, intended users, applicability (software systems and software projects of all kinds) |
5 | Conformance | The standard can be used to check conformity with ISO/IEC/IEEE 12207 and ISO/IEC/IEEE 15288 |
6 | Life-cycle data and information items | General requirements for documents and records and the difference between them. See section TODO |
7 | Generic types of information items | Overview of seven document classes and their generic content (see below) |
8 | Mapping of information items to the life cycle processes | Mapping to the standards ISO/IEC/IEEE 12207 and ISO/IEC/IEEE 15288 |
9 | Records | Specific information on records, which the standard interprets slightly differently to ISO 9000 |
10 | Specific information item (document) contents | Specific guidance for individual document types, e.g., “software architecture description” |
2. Requirements for documents
a) General requirements
In section 6, ISO/IEC/IEEE 15289 describes the general requirements for documents:
- Clarity
- Completeness
- Verifiability
- Consistency
- Modifiable
- Traceability
- Presentability
Tip
The Johner Institute recommends creating specific documentary requirements in work instructions and checklists for the document review. For example, for a software requirements specification, checking whether the runtime environment has been defined based on the hardware and operating system (including version) should be explicitly required.
b) Meta-level requirements
Additional requirements for documents at the meta-level are:
- All information/documents to be managed are identified (e.g., lists of all documents).
- The form in which the information should be presented has been defined (e.g., text, graphics, in databases).
- The status of the documents/information is identifiable.
- The information/documents are actually available to the intended stakeholders.
Additional information
ISO 15289 is not specifically about document control. Read more on the topic of document control here to comply with the requirements of ISO 13485, among others.
c) Content requirements
ISO 15289 establishes the requirements for specific documents at two levels:
- First, it assigns each document to a document class, which it calls “Generic types of information items”. It specifies the contents for each document class. This article provides an overview of the seven document classes below.
- The standard adds additional content for each specific document (e.g., a software architecture). The next section gives a specific example.
3. Document classes and generic content
a) Documents
In section 10, ISO/IEC/IEEE 15289 describes a total of 74 document types, such as Release Plan or System architecture description. The standard splits all these document types into seven document classes. However, it does not use the term document class, preferring to use the term Generic type of information items.
# | Document class | Description | Examples |
1 | Description | Representation of a planned or actual function, service, product, component, etc. | Software architecture, service catalog, operational concept |
2 | Plan | Defining when and how specific processes or activities will be carried out and by who | V&V plans, software development plan, risk management plan |
3 | Policy | Defining the top-level aims of an organization to achieve specific goals | Quality policy, risk policy (although is not specified by ISO 15289) |
4 | Procedure | Detailed definition of when and how a certain process, a certain activity or task should be carried out, including, if applicable, the tools | Instructions for use, test instructions, SOP on processing complaints |
5 | Report | Description of the results of activities | Test report, problem report, incident report, review minutes |
6 | Request | Information to ask for a response or reaction | Change request, customer satisfaction survey, RFP (request for proposal) |
7 | Specification | Requirements for a service, product or process | Contract, software requirements specification, SLA |
The standard specifies the generic content for each of these document classes. Therefore, for example, a document in the “Description” class must contain at least the following information:
- Date and status
- Scope
- References
- Notation used
- Summary
- Glossary
- Change history
Section 10 of ISO 15289 then adds additional content for each specific document. For example, a software architecture, which falls into the “Description” document class must also contain:
- One or more architectural views
- A system concept in terms of purpose and non-functional properties such as safety, interoperability and security
- Constraints (solution space limitations)
- Design decisions and justifications
Additional information
A software architecture has to implement a lot of non-functional software requirement specification requirements. ISO 25010(german) provides an overview of non-functional requirements.
b) Records
Definition
In addition to the documents and document classes (Generic types of information items), ISO 15289 also refers to Records. However, the standard understands the term records slightly differently to ISO 9000 and, as a result, also uses the terms records of data and data records.
These data records are more reminiscent of raw data in databases, registers or repositories. Records as defined by ISO 15289 contain the factual data (e.g., lists of test results in a test database) that are then turned into records as defined by ISO 9000.
A (final) test report would, therefore, already be a record.
Content
ISO 15289 also establishes the standard content for records
- Date and status of the record
- Scope
- Subject or category
- Issuing organization
- References
- Content
- Unique identification
For these records, ISO 15289 maps to the requirements of ISO/IEC/IEEE 12207 and ISO/IEC/IEEE 15288 in the same way as it does for documents.
4. Mapping to IEC 62304
Medical software developers who follow IEC 62304 can also benefit from the specifications of ISO 15289. To do this, it is necessary to map the content required by IEC 62304 onto the document types listed in ISO 15289.
Section of IEC 62304 | Required content | Documents according to ISO 15289 | Section of ISO 15289 |
5.1 | Software development plan | Development plan Project management plan Release plan Quality plan | 10.16 10.42 10.47 10.44 |
5.1 | Software risk management planning | Risk management plan (not 100% equivalent) | 10.52 |
5.1 | Documentation planning | Information management plan Information management procedure | 10.27 10.28 |
5.1 | Software configuration management planning | Configuration management plan Configuration management procedure | 10.9 10.10 |
5.2 | Software requirement analysis | System requirements specification If necessary, interface description | 10.60 10.28 |
5.2 | Installation requirements | Operational concept | 10.35 |
5.2 | Re-evaluate medical device risk analysis | Risk action request | 10.51 |
5.2 | Verify software requirements | Verification procedure Verification report | 10.73 10.74 |
Example mapping of IEC 62304 and ISO 15289
ISO 15289 is not just helpful for development documentation according to IEC 62304 section 5. It also provides an overview of additional life-cycle documents, some of which IEC 62304 only lists rudimentarily in sections 7 to 9.
5. Conclusion, summary
ISO/IEC/IEEE 15289 is a useful standard:
- It provides an overview and clarity
It provides clarity through concepts such as document classes and, as a result, reduces the feeling of drowning in a sea of documents. - It helps you to not forget anything and acts as a basis for testing
The standard lists the documents that organizations that develop software usually work with. It also provides requirements for the content of each document type. This means it can be used as a checklist for project managers, quality managers and auditors. - It provides guidance when creating documents
New companies in particular don’t know what content needs to be documented, how to divide this content across documents, and how to structure these documents. This doesn’t just apply to the development of software systems, it also applies to their operation. ISO 15289 provides specific guidance for both.
User notifications, Incident management procedures and Transition plans are examples of documents that are not directly related to development.
Established companies can also benefit from the standard, particularly if their documentation processes have become a bit chaotic over the years with redundant or superfluous content.
The Johner Institute helps medical device manufacturers whose devices contain software or are software create legally compliant software and review before audits. Get in touch with us (e.g., via our web form) to find out how you can establish lean, precise and compliant documentation that will make you shine in audits and reviews.
Auditgarant contains a comprehensive set of templates for software documentation to ensure compliance with ISO 62304 and ISO 13485 and avoid problems during medical device authorizations.