Notified bodies at the limit: How to get through the IVDR conformity assessment more easily
You need a conformity assessment by a notified body and have been waiting for a response for months or have possibly even been rejected? You are not alone in this!
Since the new regulation came into force, ten times more IVDs than before require testing and assessment by a notified body, while at the same time, there are fewer bodies designated for the IVDR! The resulting capacity problems are at the expense of many manufacturers.
In clear terms, notified bodies may reject or move a manufacturer's application to the back of the queue if there is uncertainty about the overall effort or concerns about a positive outcome.
Increase your chance of direct acceptance with the help of our IVDR Readiness Certificate!
Minimize the risk of rejection by a notified body - and thus jeopardizing your market access

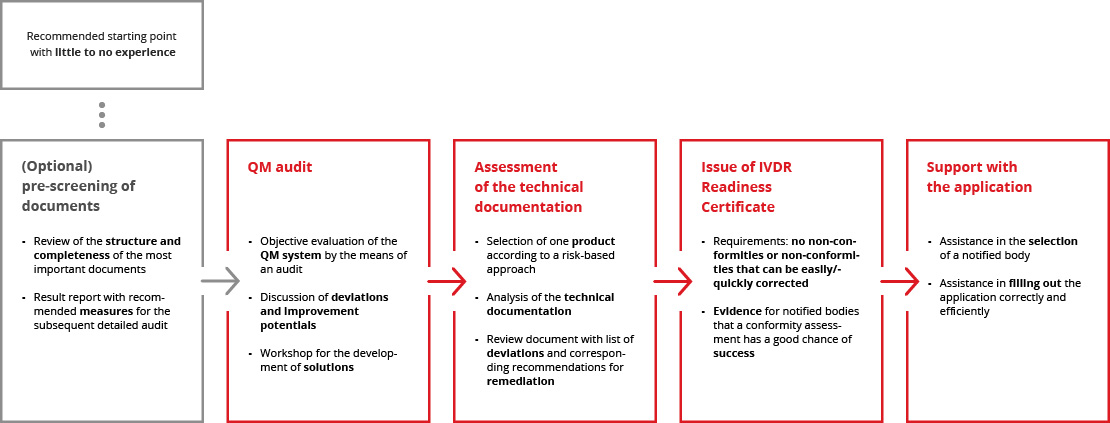
We perform a mock conformity assessment of your QM system and an exemplary technical documentation. If both are of appropriate quality, we issue an IVDR Readiness Certificate that you can present to a notified body.
The independent audit by our experienced experts provides notified bodies with the assurance that no significant gaps in the documentation or process landscape are to be expected. The IVDR Readiness Certificate thus serves as proof that a conformity assessment can be carried out efficiently and in a resource-saving manner.
Save time and effort in the application process, so nothing stands in the way of a smooth testing and assessment
The application to a notified body follows strict regulatory requirements and can be time-consuming due to its scope and complexity. Our team is happy to support you and ensure that the application is completed correctly and that the submission is as efficient as possible.
The path to IVDR Readiness Certification
Can we help you with this?
Feel free to get in touch via our contact form to request a free consultation. One of our business analysts will get back to you as soon as possible to check in a joint preliminary discussion on whether and how we can best help you.
