Manufacturing and Marketing Personal Protective Equipment (PPE) in Compliance with the Legislation
Someone who wants to place personal protective equipment (PPE) on the market has to comply with different legislation to someone who wants to place medical devices (MD) on the market. It is therefore important that the applicable regulations for products such as gloves are identified and followed.
This article describes how products are classified as either personal protective equipment or medical devices. It provides an overview of the regulations and sets out a pathway for the quick and legally compliant manufacture and marketing of personal protective equipment. This way, legal risks and unnecessary expenses can be avoided.
1. Personal protective equipment (PPE) versus medical devices
a) The intended purpose is decisive
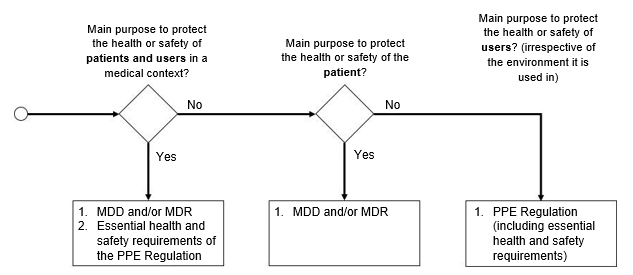
Whether a products is classified as personal protective equipment or not depends on the intended purpose of the product:
- If the device is intended exclusively for the protection of the user (the person wearing it) against one or more health and safety hazards, then the device is classified as personal protective equipment.
- Whereas if a product is designed to protect patients, it is considered a medical device.
If a product can be used for both intended purposes, it is both a medical device and personal protective equipment.
b) Examples
The following table gives some examples that show how the intended purpose is decisive when it comes to the question of whether a product should be classified as a medical device or as personal protective equipment (or both).
Product | Medical device | Personal protective equipment |
Gloves | Surgical gloves, examination gloves | Protective gloves (e.g., for use in medical laboratories) |
Clothing | Surgical clothing (gowns and caps), clothing to protect against radiation (e.g., gonad shields) for patients | Clothing to protect against radiation for users |
Mouth protection | Mouth protection with a medical purpose | Mouthguards for boxers |
Masks | Respirators with a medical purpose | Dust masks |
Glasses | Corrective glasses (including those with light protection) | Sunglasses, protective goggles (including those with customized lenses), protective goggles for specific professions (e.g., welding goggles) |
Laser glasses | Laser safety glasses for patients | Laser safety goggles for users |
Bandages | Therapeutic bandages | Sports bandages for prevention |
Helmets | Protective helmet for craniectomy, epilepsy helmet | Bicycle helmet, motorcycle helmet, sport helmet |
The classification is not based on functionality or the type of device, it is based on the intended purpose!
c) Definitions
Regulation (EU) 2016/425 (“PPE Regulation”) defines “personal protective equipment” in Article 3.
“(a) | equipment designed and manufactured to be worn or held by a person for protection against one or more risks to that person's health or safety;
(b) | interchangeable components for equipment referred to in point (a) which are essential for its protective function;
(c) | connexion systems for equipment referred to in point (a) that are not held or worn by a person, that are designed to connect that equipment to an external device or to a reliable anchorage point, that are not designed to be permanently fixed and that do not require fastening works before use;
EU Regulation 2016/425 Article 3
2. Regulatory requirements for personal protective equipment (PPE)
a) Overview of Regulation 2016/425
Manufacturers of personal protective equipment must comply with Regulation (EU) 2016/425 on personal protective equipment. This regulation replaced Directive 89/686/EEC in 2016.
This “PPE Regulation” is short compared to the MDR (82 pages compared to 263). Both regulations use similar concepts:
- Manufacturers must complete a conformity assessment procedure to demonstrate the conformity of the PPE.
- In the case of the PPE Regulation, the choice of conformity assessment procedure depends on the type of PPE. Just like the classification for medical devices, the category of PPE determines the possible procedures.
- Both regulations establish requirements for the products. In the PPE Regulation, these are called the “essential health and safety requirements”, and in the MDR, they are called the “general safety and performance requirements.”
b) Requirements for products that are PPE and(!) medical devices
The Medical Device Directive (MDD) answers the question of whether a medical device at the same time can also be considered personal protective equipment as follows:
“6. Where a device is intended by the manufacturer to be used in accordance with both the provisions on personal protective equipment in Council Directive 89/686/EEC and this Directive, the relevant basic health and safety requirements of Directive 89/686/EEC [comment: old directive] shall also be fulfilled.”
Medical Device Directive 93/42/EEC Article 1
Interestingly, no official website currently refers to this passage.
The Medical Device Regulation MDR, (2017/245) does not establish this requirement. This is understandable, since the MDR establishes higher requirements for medical devices than the PPE Regulation does for PPE. It (largely) covers the “essential health and safety requirements.”
The following flowchart will help you identify the applicable regulations:
In the case of PPE that is also0 a medical device, manufacturers must also comply with the Medical Device Directive and/or the Medical Device Regulation.
c) Standards
Manufacturers can use product-specific harmonized standards to prove conformity with the legal requirements. Compliance with these standards is not mandatory, provided the specific health and safety requirements are met. Nevertheless, the Johner Institute recommends using them.
If manufacturers use standards, they should make sure they are using the currently valid versions, including their annexes.
The following table provides an overview of the classification of various products with applicable regulations and standards.
Product | Classification | Regulations/standards |
Safety goggles | Regulation (EU) 2016/425 (PPE) | EN 166, |
Face shield (full face mask) | Regulation (EU) 2016/425 (PPE) | EN 166, |
Particle filtering half face mask (e.g., FFP2 & FFP3 masks) | Regulation (EU) 2016/425 (PPE) | EN 149 (or temporarily equivalent standards, such as NIOSH N95 (USA), KN95 (CHN), P2(AUS/NZL), DS (JPN), 1st Class (KOR) |
Surgical mask | Directive (EU) 93/42/EEC or Regulation (EU) 2017/745 (medical device) | EN 14683 (or temporarily equivalent standards, such as ASTM) |
Surgical clothing | Directive (EU) 93/42/EEC or Regulation (EU) 2017/745 (medical device) | EN 13795, |
Protective clothing | Regulation (EU) 2016/425 (PPE) | EN 13795, |
Disposable gloves | Regulation (EU) 2016/425 (PPE) or (in addition) Directive (EU) 93/42/EEC or Regulation (EU) 2017/745 (medical device), depending on the intended purpose | EN 374, |
d) Technical documentation
Manufacturers and distributors of personal protective equipment (PPE) must create technical documentation, as described in Annex III of Regulation 2016/425. The regulation requires manufacturers to document at least the following:
- a complete description of the PPE and of its intended use;
- an assessment of the risks against which the PPE is intended to protect;
- a list of the essential health and safety requirements that are applicable to the PPE;
- design and manufacturing drawings and schemes of the PPE and of its components, sub-assemblies and circuits;
- the descriptions and explanations necessary for the understanding of the drawings and schemes referred to in point (d) and of the operation of the PPE;
- the references of the harmonised standards referred to in Article 14 that have been applied for the design and manufacture of the PPE. In the event of partial application of harmonised standards, the documentation shall specify the parts which have been applied;
- where harmonised standards have not been applied or have been only partially applied, descriptions of the other technical specifications that have been applied in order to satisfy the applicable essential health and safety requirements;
- the results of the design calculations, inspections and examinations carried out to verify the conformity of the PPE with the applicable essential health and safety requirements;
- reports on the tests carried out to verify the conformity of the PPE with the applicable essential health and safety requirements and, where appropriate, to establish the relevant protection class;
- a description of the means used by the manufacturer during the production of the PPE to ensure the conformity of the PPE produced with the design specifications;
- a copy of the manufacturer's instructions and information set out in point 1.4 of Annex II;
- for PPE produced as a single unit to fit an individual user, all the necessary instructions for manufacturing such PPE on the basis of the approved basic model;
- for PPE produced in series where each item is adapted to fit an individual user, a description of the measures to be taken by the manufacturer during the fitting and production process to ensure that each item of PPE complies with the approved type and with the applicable essential health and safety requirements.”
e) Essential health and safety requirements
Personal protective equipment must meet the “essential health and safety requirements.” These requirements are detailed in Annex II of the regulation. Examples of these requirements are:
- Compliance with design principles
- Ensuring comfort and effectiveness
- Ensuring the innocuousness of the PPE
- Availability of instructions and information
and other additional requirements for the different types of PPE or in the case of special risks.
Therefore, the PPE Regulation (like the medical device regulations) requires that the “state of the art” is taken into account. This state of the art is reflected in the latest versions of the aforementioned harmonized standards.
f) Conformity assessment procedure
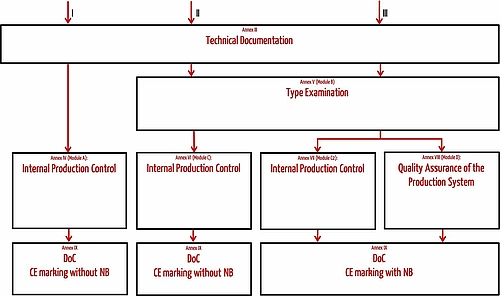
Manufacturers must prove that each item of PPE meets the requirements for personal protective equipment through a conformity assessment procedure.
The scope of this conformity assessment procedure depends on the product category.
Personal protective equipment of all categories
Product-specific technical documentation that complies with Annex III must be created for all items of PPE.
Category I personal protective equipment
Manufacturers of category I PPE must also perform an internal production control according to Annex IV (Module A).
Category II personal protective equipment
Category II PPE must undergo a type examination according to Annex V (Module B) carried out by a notified body. The notified body checks that the production type conforms with the regulatory requirements. The manufacturer ensures the continued quality and safety of their PPE through a production control according to Annex VI (Module C).
Category III personal protective equipment
Category III PPE can achieve the declaration of conformity in two different ways.
- Option 1: In addition to the type examination according to Annex V (Module B), conformity is demonstrated through an internal production control with supervised product checks at random intervals (Annex VII, Module C2).
- Option 2: In addition to the type-examination according to Annex V, conformity is demonstrated based on quality assurance of the production process (Annex VIII, Module D).
For both options, the manufacturer must involve a notified body and their identification number of the notified body must be displayed on the PPE next to the CE marking.
There are 18 notified bodies in Germany for Regulation 2016/425. A list of all notified bodies for this regulation can be found on the EU’s website.
g) Categorization / classification
Personal protective equipment is split into categories on the basis of Annex I of the PPE Regulation.
Category I includes exclusively the following minimal risks:
- superficial mechanical injury;
- contact with cleaning materials of weak action or prolonged contact with water;
- contact with hot surfaces not exceeding 50 °C;
- damage to the eyes due to exposure to sunlight (other than during observation of the sun);
- atmospheric conditions that are not of an extreme nature.
Category II includes risks other than those listed in Categories I and III;
Category III includes exclusively the risks that may cause very serious consequences such as death or irreversible damage to health relating to the following:
- substances and mixtures which are hazardous to health;
- atmospheres with oxygen deficiency;
- harmful biological agents;
- ionising radiation;
- high-temperature environments the effects of which are comparable to those of an air temperature of at least 100 °C;
- low-temperature environments the effects of which are comparable to those of an air temperature of – 50 °C or less;
- falling from a height;
- electric shock and live working;
- drowning;
- cuts by hand-held chainsaws;
- high-pressure jets;
- bullet wounds or knife stabs;
- harmful noise.
A glove that is intended to protect someone while they do some gardening would be placed in category I. A glove intended to protect a butcher against knife wounds (chain mail glove) must meet the requirements of category III.
Further information
If these rules are not enough for you, we recommend reading the European “PPE Regulation (EU) 2016/425 Guidelines”. The guideline comments on the entire PPE Regulation in an easy-to-understand way and provides a lot of information on implementation. Notes on the categorization can be found from page 82 onward.
3. FAQs in the context of the coronavirus pandemic
As a result of the current coronavirus pandemic, there is a global shortage of personal protective equipment. There is, in particular, a lack of products such as respirators, disposable gloves and disinfectants. This shortage has dangerous consequences:
- Dozens of practices have had to close in Germany alone due to an acute shortage of materials.
- Physicians and nursing staff are at high risk due to the lack of material. The Italian medical association FNOMCeO has set up its own website dedicated to the more than 50 Italian physicians who have died so far.
All the talk is of a “dramatic state of emergency” and there is no improvement in sight in the short term. It is, therefore, a gleam of hope that a lot of companies and private individuals want to contribute to combating the crisis.
The Johner Institute has come across a lot questions. The answers to the most frequently asked questions can be found below.
a) How should I classify protective clothing: as PPE or as an MD?
As described above, a product is classified as a medical device or personal protective equipment based on its intended purpose.
In a Commission Recommendation, the EU writes:
“Disposable and re-usable face masks ensuring protection against particulate hazards, disposable and re-usable coveralls, gloves and eyewear protection, which are used for prevention and protection against harmful biological agents such as viruses are products falling within the scope of the Regulation (EU) 2016/425. […]
Surgical masks, examination gloves and some types of gowns are products falling within the scope of Directive 93/42/EEC and of Regulation (EU) 2017/745.”
EU Commission Recommendation
Non-invasive medical devices (medical gloves and other medical protective clothing) are in class I, unless specific rules apply.
b) Is there a simplified procedure for placing products on the market given the emergency situation?
Existing exemptions
Member States may grant exemptions from the conformity assessment procedures in their own territory. This requires a duly substantiated application for the placing on the market and putting into service of individual medical devices. Furthermore, the use of the device must be in the interest of health protection.
According to Section 11 of the German Medical Devices Act, medical devices can be authorized for a limited period of time without a conformity assessment procedure if a duly justified application is submitted to the higher federal authority.
EU Commission Recommendation
On March 13, 2020, the EU Commission published a Commission Recommendation on conformity assessment and market surveillance procedures within the context of the COVID-19 threat. The Commission makes the following recommendation for the authorities:
“[...] Accordingly, to address the shortage of PPE necessary in the context of the COVID-19 outbreak, where non-CE marked PPE are intended to enter the EU market, the relevant market surveillance authorities should evaluate the products and, if they are found to be compliant with the essential health and safety requirements laid down by the relevant Regulation should take measures allowing the placing of such PPE on the Union market for a limited period of time or while the conformity assessment procedure with the notified body is being carried out.”
EU Commission Recommendation
Personal protective equipment that is authorized in other countries, such as the USA, Canada or Australia, can also be temporarily placed on the market. For detailed information on whether a device can be placed on the market, manufacturers should contact the relevant authority.
The Commission Recommendation also states that economic operators throughout the supply chain should deploy all the measures at their disposal to ensure the quick supply of PPE and medical devices. For example, notified bodies should prioritize and swiftly process these applications.
NB!
It is still the case that PPE and medical devices may only be placed on the market without a conformity assessment procedure if the devices continue to adequately protect the health and safety of users. PPE or medical devices placed on the market in this way may only be issued to healthcare workers and only for the duration of this threat
For personal protective equipment, there are already three notified bodies working based on the test method described. The Central Authority of the Federal States for Safety Technology (ZLS, Zentralstelle der Länder für Sicherheitstechnik) has listed them on its website.
PPE manufactured in accordance with other technical solutions and for which no harmonized standards were used must meet the requirements detailed in the WHO recommendations as well as the essential health and safety requirements.
c) What should I do to place my product on the market?
If you want to place personal protective equipment on the European market, you need to do the following:
- First of all, document the intended purpose of the product.
- Qualification: Then decide whether the product is a medical device or personal protective equipment.
- Regulations: Based on this decision, identify which regulation is applicable: The MDR or MDD or/and the PPE Regulation
- Choose the conformity assessment procedure.
- Design the product and create the technical documentation for it in accordance with the “general safety and performance requirements” (MDR) or the “essential health and safety requirements” (PPE Regulation). Refer to the relevant (harmonized) standards to demonstrate compliance.
- Prepare the declaration of conformity without the involvement of a notified body (for class I medical devices and category I and II PPE) or with the involvement of a notified body (category III PPE). Alternatively, in the context of COVID-19, choose the “simplified placing on the market procedure” described above.
- Place your product on the market, i.e., sell it or give it away.
d) What specific rules are there for respirators?
As there is a particular shortage of respirators, we will take a closer look at this particular case.
Classification of masks
First of all, we have to differentiate between two types of masks:
- Respirators (FFP1, FFP2, FFP3)
- Surgical masks
The following table explains the differences between these two types of masks:
Product | Surgical mask | Respirator |
Intended purpose | Protecting patients from infectious microbes during examinations or operations as well as, in certain situations, protecting the wearer against splashes from potentially contaminated fluids. In specific situations, such as epidemics or pandemics, medical face masks can also be worn by patients and other people to reduce the risk of infection. | Protecting the user against both solid and liquid aerosols (e.g., viruses, bacteria, dust). |
Qualification | These masks are considered medical devices. | Respirators (FFP1, FFP2, FFP3) are classified as personal protective equipment. |
Applicable regulations | The requirements of the MDD and/or MDR (Regulation (EU) 2017/745) must be met. | The aforementioned requirements, in particular Regulation (EU) 2016/425, must be met. |
Classification, categorization | The classification rules can be found in Annex IX of the MDD and in Chapter V of the MDR. Surgical masks are generally classified as class I. This blog post describes how class I medical devices can be placed on the market. | Protective equipment designed to protect against harmful biological agents such as viruses must be put in category III as its use relates to “risks that may cause very serious consequences such as death or irreversible damage to health.” |
Involvement of a notified body necessary | No | Yes |
Properties | High resistance to fluid, good breathability | Good breathability, mostly through an exhalation valve, metal plate for positioning the mask on the nose, reusable if necessary |
Regulations & standards | Type I, II or IIR according to EN 14683 or ASTM F2100 level 2 or level 3 or equivalent | FFP1, FFP2, FFP3 according to EN 149 or “N95” respirator according to the FDA class II, as per 21 CFR 878.4040, and CDC NIOSH, or equivalent standards |
The options described above for quick placing on the market are also available for these devices.
Importing of masks
When importing this type of personal protective equipment from countries outside the EEA, it must be checked whether the products have already been placed on the European market and whether the products have already gone through a conformity assessment procedure, or whether they have CE marking (for PPE, with the identification number of a notified body).
Otherwise, the importer (called an “Einführer” in the German version) must place the PPE on the market through a conformity assessment procedure. The importer's obligations are set out in Article 10 of the PPE Regulation and Article 13 of the Medical Device Regulation.
NB!
Be wary of counterfeit products when importing! In particular, check that the declarations of conformity are authentic. There are countless counterfeit respirators circulating. In any case, always sign a quality assurance agreement (QAA) with the supplier and obtain legal advice if necessary.
Masks that can be placed on the market in the USA, Canada, Australia, Japan or China, for example NIOSH N95 masks, can temporarily be placed on the European market without CE marking.
The Federal Institute for Occupational Safety and Health (BAuA) has created an information sheet on internationally used masks that explains the differences between the products. The manufacturer 3M has published a comparison of the different standards that apply to respirators. Anyone interested in importing respirators should read the documents.
Free standards
Two standards, in particular, are relevant for masks:
DIN EN 14683 Medical face masks for medical devices, and DIN EN 149 for personal protective equipment.
Product | DIN EN 14683 | DIN EN 149 |
Scope | Protecting patients from infectious microbes during examinations or operations as well as, in certain situations, protecting the wearer against splashes from potentially contaminated fluids. In specific situations, such as epidemics or pandemics, medical face masks can also be worn by patients and other people to reduce the risk of infection. | Protecting the user against both solid and liquid aerosols (e.g., viruses, bacteria, dust). |
Classification | Classification of masks according to performance: | Classification of masks according to performance: |
Type of testing | Laboratory testing | Laboratory testing and practical performance testing |
Main testing criteria | Bacterial filtration efficiency | Filter efficiency & filter permeability |
Test expenditure | Medium | High |
The European standardization organizations have made COVID-19-relevant standards, including DIN EN 14683 and DIN EN 149, available free of charge until further notice:
- These are available from Beuth Verlag free of charge.
- The Association for the Advancement of Medical Instrumentation (AAMI) has also made three COVID-19-relevant standards available free of charge.
Sale of non-certified masks or face protection
The city council in Jena has made it compulsory to wear masks in public. It released the following statement:
“Sew important face masks for yourself and for other people to stem the spread of the virus – any mask is better than no mask.”
Jena city council
There is no data at this point as to if, and when, masks really help.
NB!
Beware of accidentally placing a product on the market as PPE or a medical device!
If you manufacture masks yourself and place them on the market (for example, sell them or give them away), they must not be classified as PPE or medical devices due to their intended purpose. Otherwise, the products would have to undergo a conformity assessment procedure and you would have to be able to prove that the product complies with the requirements.
If you place medical devices or personal protective equipment on the market without a conformity assessment procedure, these products cannot be marketed and you may be liable to prosecution. Warnings and more are imminent.
- Therefore, when selling masks that you have sewn yourself, be careful not to give the impression that the masks are personal protective equipment or medical devices.
- Do not make any advertising statements regarding their performance, for example, claims that the masks protect against bacteria, viruses, dust or similar substances, or that your masks prevent infection.
- Avoid terms such as “respirator”, “mouth and nose protector” and “protection” in general.
- Please expressly point out that this is neither personal protective equipment or a medical device.
Possible terms you could use are “mask”, “face mask”, “DIY mask”, or “makeshift surgical mask” for private use.
The Federal Institute for Drugs and Medical Devices (BfArM) has issued an important notice on this issue and it can be found here.
The same applies to visors such as face protectors and shields.
e) What special regulations has the FDA established?
The FDA has also recognized the urgent situation and published various guidance documents to facilitate quick product authorization.
The FDA also classifies masks as either medical masks or personal protective equipment (PPE).
Personal protective equipment is regulated in 21 Code of Federal Regulations.
Medical face masks and respirators, which the FDA categorizes as class II, are defined by the FDA as follows:
“Face masks and respirators are devices when they are intended for a medical purpose, such as prevention of infectious disease transmission (including uses related to COVID-19). Face masks and respirators are not devices when they are intended for a non-medical purpose, such as for use in construction. When considering whether these products are intended for a medical purpose, among other considerations, FDA will look at whether:
1) they are labeled or otherwise intended for use by a health care professional;
2) they are labeled or otherwise for use in a health care facility or environment; and 3) they include any drugs, biologics, or anti-microbial/anti-viral agents.”
FDA
Personal protective equipment, such as face masks and respirators, are classified differently in the USA than in Europe. Protective equipment falls under the definition of a medical device more often, namely as soon as the equipment is used in a medical setting.
The FDA has published a guidance document entitled “Enforcement Policy for Face Masks and Respirators during the Coronavirus Disease (COVID-19) Public Health Emergency”, which is worth reading and which illustrates the classification of face masks respirators.
In this guidance document, the FDA states that individuals, including healthcare professionals, may use masks and respirators that have not been cleared by the FDA. The FDA would have no objection to the distribution and use of masks or respirators that have not been authorized, provided there are no alternatives available. The document also establishes a minimum set of requirements.
Furthermore, surgical masks may be distributed and used for the duration of the public health emergency without the surgical masks having previously been the subject of a premarket notification under section 510(k) of the FD&C Act and 21 CFR 807.81. It is important that the masks do not represent an “undue risk”, which the document goes on to explain in more detail. The Center for Disease Control and Prevention (CDC) has published a list of respirators that use a different standard but can be used temporarily.
In addition, strategies to improve the supply of N95 masks are detailed.
d) Where can I find further information?
You should pay attention to the following information that is relevant in the context of the corona crisis:
- FAQ on the marketing of personal protective equipment from the Federal Institute for Occupational Safety and Health (BAuA) as the competent German higher federal authority for the current situation
- The EU's information page on placing PPE on the market with regard to the coronavirus situation
- Document on possible measures for the resource-saving use of masks in connection with coronavirus, Robert Koch Institute (RKI)
- The European Commission's FAQ on PPE conformity assessment procedures with regard to COVID-19
- List of ZLS approved laboratories
- The Johner Institute is also offering free support as part of our micro-consulting service.
4. Support from the Johner Institute
a) Medical devices
The Johner Institute can help manufacturers place their medical devices on the market quickly and without complications.
- Establishing a regulatory strategy
- Selecting notified bodies
- Selecting a testing laboratory according to DIN EN 14683
- Preparing the technical documentation
- Setting up and testing a QM system
- Testing biocompatibility
- Carrying out post-market surveillance
- And much more
b) Personal protective equipment
The Johner Institute can help manufacturers of personal protective equipment demonstrate the biocompatibility of their products and identify companies that can help with the next steps.
Get in touch with us.
5. Conclusion, summary
The “corona crisis” has made almost everyone aware of how important personal protective equipment (PPE) is. The legal requirements that PPE manufacturers must comply with are clearly described in EU Regulation 2016/425.
These requirements are similar in structure to the requirements contained in the MDR. They are, however, less extensive.
Manufacturers should use standards to demonstrate the conformity of their PPE products. They also have to go through a conformity assessment procedure.
However, exceptions are allowed in the context of the current coronavirus pandemic. The risk-benefit assessment has seldom been so important.