Medicinal product or (substance-based) medical device?
The classification of whether a device is a medicinal product or a substance-based medical device has far-reaching regulatory consequences. This classification is so demanding that there are regular disputes with authorities and notified bodies, and in 2023, even the European Court of Justice had to rule on the matter.
This article helps manufacturers, authorities, and notified bodies to "qualify" a device. It addresses the ECJ judgment mentioned above, as well as regulatory requirements and guidance. You will learn what a presentation medicinal product is and in which cases the national courts will still be asked.
1. Summary for readers in a hurry
- A device that achieves its intended medical purpose (almost) exclusively physically counts as a (substance-based) medical device.
- A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product.
If the main physical mode of action is not clearly proven, the device must be treated as a medicinal product.
Further information
Here, you will find a compact overview of the regulatory requirements for substance-based medical devices.
2. Basics
a) What are substance-based medical devices?
Definition
Even though the MDR recognizes the existence of "devices consisting of substances or combinations of substances" with classification rules 3 and 21 and provides a legal framework, it still fails to define substance-based medical devices clearly. For this reason, the MDCG provides in document MDCG 2022-5 and provides the following definition:
Definition „Substance-based medical device“
“A substance-based medical device is a medical device which:
· is composed of substances that are permitted in a medical device, and
· does not achieve its main intended effect by pharmacological, metabolic or immunological means.”
Quelle: MDCG 2022-5 / Part: 3.1
Substance-based medical devices, therefore, consist of substances and are similar to medicinal products in their presentation and dosage form. However, they do not achieve their main intended effect via a pharmacological, metabolic, or immunological mechanism but primarily by physical means.
Examples
Examples of substance-based medical devices include:
- Saline nasal or throat sprays
- Mucous membrane-moistening syrups, throat sprays, or lozenges
- Artificial tears
- Oral products to neutralize gastric acid
- Devices with a defoaming effect for gastrointestinal complaints caused by gas
b) What are medicinal products?
Definition
The definition for medicinal products is provided by Directive 2001/83/EC:
Definition medicinal product
“a) Any substance or combination of substances presented for treating or preventing disease in human beings.
b) Any substance or combination of substances which may be administered to human beings with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in human beings is likewise considered a medicinal product.”
Source: Directive 2001/83/EC Article 1(2)
This definition consists of two parts: part a) relating more to the presentation or get-up of a device (presentation medicinal product) and part b) relating to the function (functional medicinal product). Thus, a device is a medicinal product if it falls under part (a), part (b), or both parts of the definition.
Further information
The terms "presentation medicinal product" and "functional medicinal product" are not part of the definition of the term but are rather common terms for the different preparations according to the BfArM. This distinction is primarily used for delineation in case law.
- Accordingly, presentation medicinal products are those whose designation or presentation (advertising) gives the average informed consumer the impression that they are intended to cure or prevent human diseases, irrespective of their actual mode of action.
- On the other hand, functional medicinal products have a significant influence on the physiological functions of the human organism through their effect.
Consequently, this means that only functional medicinal products are, by definition, characterized by an actual (scientifically verifiable) pharmacological, immunological, or metabolic mode of action.
Examples
Examples of medicinal product groups are:
- Analgesics
- Anticoagulants
- Insulin
- Cytostatics
- x-ray contrast media
c) Distinction between medicinal products and medical devices
Since substance-based medical devices also consist of substances and are intended for use in the treatment, alleviation, or prevention of human disease, a clear demarcation between medical devices and (presentation) medicinal products is often not readily possible.
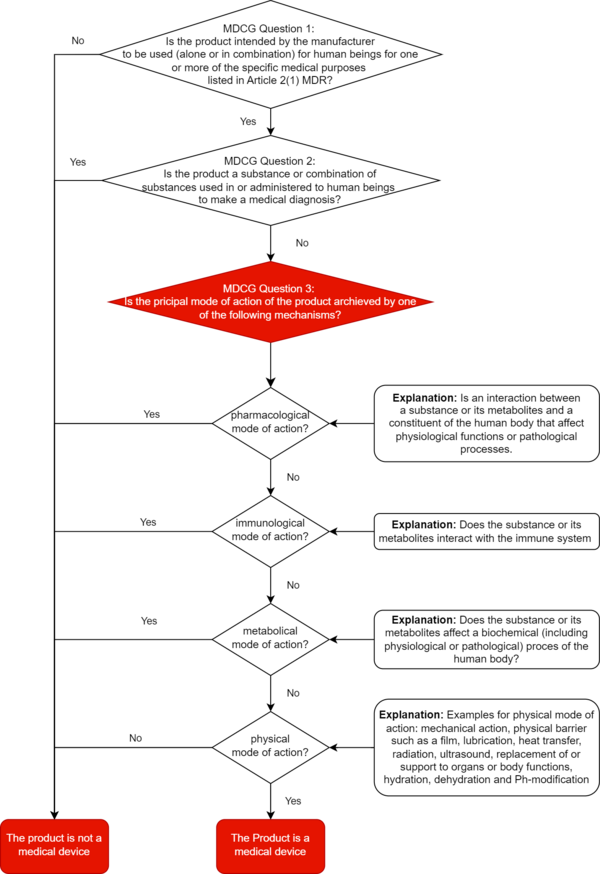
Products for which it is not clear from the outset whether they are medical devices or medicinal products are referred to as borderline products in accordance with MDCG 2022-5. The principal mode of action is decisive in the demarcation of these devices.
Important!
A device cannot fall under both legal regulations, i.e., it cannot be a medicinal product and a medical device at the same time.
In the case that a device can fall under both the definition of "medical device" and the definition of " medicinal product," the device is a medicinal product according to the so-called doubt rule:
“In cases of doubt, where, taking into account all its characteristics, a product may fall within the definition of a ‘medicinal product’ and within the definition of a product covered by other Community legislation the provisions of this Directive shall apply.”
Source: Directive 2001/83/EC Article 2(2)
3. The momentous ECJ judgment
a) The facts
At the request of the Federal Administrative Court, the European Court of Justice dealt with various questions on the distinction between substance-based medical devices and medicinal products. The starting point was legal disputes concerning the qualification of preparations declared as medical devices for use in the nasal mucosa, for which, however, according to the German Federal Institute for Drugs and Medical Devices (BfArM), prior authorization as a drug was required.
b) The ECJ's decision
The ECJ clarifies once again in its judgment of January 19, 2023:
- The main intended effect of substance-based medical devices is predominantly physical.
- In contrast, medicinal products act in a pharmacological, metabolic, or immunological manner.
The rule of doubt, according to Article 2 (2) of Directive 2001/83/EC, applies equally to functional and presentation medicinal products.
However, suppose the main intended effect cannot be clearly explained based on the available scientific evidence for a device. In that case, the latter is to be classified neither as a medical device nor as a medicinal product - according to the ECJ.
Borderline products
How are these borderline products to be classified in this case in the future?
According to the ECJ judgment, the possible classification as presentation medicinal products remains, because according to the definition, these are products that are not intended to cure or prevent human diseases based on their proven mode of action but based on their presentation and their advertised properties.
“Where the principal mode of action of a product is not scientifically established, that product cannot meet the definition of the concept of ‘medical device’, within the meaning of Directive 93/42, as amended by Directive 2007/47, or that of ‘medicinal product by function’, as referred to in Directive 2001/83, as amended by Directive 2004/27.
It is for the national courts to assess, on a case-by-case basis, whether the conditions relating to the definition of the concept of ‘medicinal product by presentation’, as referred to in Directive 2001/83, as amended by Directive 2004/27, are satisfied.”
ECJ judgment
In this context, the ECJ attributed a higher level of consumer protection to the Directive on medicinal products (Directive 2001/83/EC), since the placing on the market of medicinal products requires the prior granting of an authorization by the authorities.
4. Possible consequences for manufacturers
The ECJ judgment could have significant consequences for the manufacturers of many substance-based medical devices on the market. Mistakes in qualification and classification that are recognized too late can cost time, effort, and money. In addition, manufacturers risk legal disputes.
In the absence of scientific evidence that the main intended effect in or on the human body is achieved by physical means and not by pharmacological, immunological, and metabolic means, there is a risk that these borderline products will regularly have to be classified as presentation medicinal products and consequently be subject to the legal regulation for medicinal products.
Scientific proof is crucial
In order to prevent costly and time-consuming legal disputes on delimitation, it is important to prove the effect of the product scientifically. The following applies here:
- Only a product that fulfills its intended use through exclusively physical mechanisms of action falls under the definition of "medical device" as defined by the MDR.
- Suppose substances are used that can basically have a pharmacological, immunological, or metabolic effect. In that case, special care must be taken: In this case, it must be demonstrated that these substances play no role in fulfilling the main intended effect of the product (e.g., flavoring and preservatives).
- A product that achieves its main intended effect solely by pharmacological, immunological, or metabolic means falls within the definition of "medicinal product" as defined in Directive 2001/83/EC.
- If a product can fall under both the definition of "medical device" and the definition of "medicinal product," the rule of doubt, according to Article 2 (2) of Directive 2001/83/EC, applies, and the device is classified as a medicinal product.
- If the mode of action is unclear or scientific evidence is lacking, the product falls neither under the definition of "medical device" nor under the definition of "medicinal product."
- Suppose a product corresponds to a medical device in its presentation but uses a pharmacological, metabolic, or immunological effect of an ingredient to achieve the intended use. In that case, the device must be approved as a medicinal product.
5. Delimitation by means of MDCG 2022-5
The Medical Devices Coordination Group clarified in its guidance on the demarcation between medical devices and medicinal products even before the ECJ ruling was announced: A product cannot be classified as a medical device unless it is clearly demonstrated that its main intended effect is not based on a primarily pharmacological, immunological, or metabolic mode of action.
a) The main intended effect decides
The decisive criterion for differentiating substance-based medical devices from medicinal products is the main intended effect of a device.
The MDCG document defines the main intended effect (as defined in Article 1(6)(b) of the MDR) as the principle by which the device achieves its main intended effect, i.e., pharmacological, immunological, metabolic, physical, or other.
This effect must be demonstrated objectively and in accordance with the latest scientific knowledge. The manufacturer's claims for its device are irrelevant.
However, the MDCG 2022-5 guidance document does not explain what this evidence might look like in concrete terms. This poses significant challenges for manufacturers of substance-based medical devices!
b) Pharmacological, metabolic, immunological, physical
At least: In this context, the MDCG sets out in section 1.2.2 what it understands by "pharmacological," "immunological," "metabolic," and "physical," and gives examples.
‘Pharmacological means’ is understood as an interaction typically at a molecular level between a substance or its metabolites and a constituent of the human body which results in initiation, enhancement, reduction or blockade of physiological functions or pathological processes
‘Immunological means’ is understood as an action initiated by a substance or its metabolites on the human body and mediated or exerted (i.e., stimulation, modulation, blocking, replacement) by cells or molecules involved in the functioning of the immune system.
‘Metabolic means’ is understood as an action of a substance or its metabolites which involves an alteration, including stopping, starting, or changing the rate, extent, or nature of a biochemical process, whether physiological or pathological,
Source: MDCG Paragraph 1.2.2
Examples of physical means by which a device achieves its principal intended effect include:
- mechanical action
- physical barriers, e.g., building up a film
- heat transfer
- radiation
- ultrasound
- replacement and support of organs and body functions
- hydration or dehydration
- change of the pH-value
The MDCG document provides manufacturers with a decision diagram for correct qualification. We have expanded this decision tree for you to include the definitions of pharmacological, immunological, metabolic, or physical principal mode of action.
6. Instructions for manufacturers
a) Qualification and classification in everyday practice
With the introduction of Rule 21 of the MDR, Annex VIII, many substance-based medical devices that were regularly classified as Class I under the MDD will be reclassified higher. This makes the involvement of a notified body necessary.
The justification for qualification as a medical device must already be submitted for the application to a notified body.
For the qualification as a substance-based medical device, the mechanism of action is decisive.
Borderline products are difficult to distinguish. Without sufficiently substantiated qualification, manufacturers risk classification of these products by national courts and thus delays in placing them on the market.
b) Tips
Tip 1: Qualify and classify the device at an early stage
Start in time! The first step is to qualify and classify a product as a (substance-based) medical device. Only then should you ensure conformity with the MDR's requirements.
When qualifying substance-based medical devices, consider the specific classification rules 3 and 21, among others.
Note
Mistakes in qualification and classification that are recognized too late can cost you time, effort, and money. In addition, you risk legal disputes.
We support you with an expert opinion in qualifying your device and collecting and documenting the necessary evidence.
Contact us right away.
Tip 2: Check evidence documents
If you know the main intended effect of your device, you should check the modes of action of the individual substances that contribute to it. Only devices with physical principal modes of action are covered by the MDR.
Make sure you have all the evidence to support the principal mode of action. For medical devices, this must be physical.
Tip
Use the definitions of terms for the principal mode of action in MDCG 2022-5 as a guide.
7. Conclusion
Although the January 2023 ECJ judgment did not consider MDCG document 2022-5 in its rulings, it confirmed the requirements for the distinction between medical devices and medicinal products.
Accordingly, the decisive criterion is a product's principal mode of action to fulfill its intended use.
The MDCG defines what it means by pharmacological, immunological, and metabolic principal mode of action, thus underpinning the obligation of manufacturers to exclude these modes of action in medical devices.
In order to qualify a substance-based medical device as a medical device, the physical mode of action must also be confirmed. Only this proof refers to the applicability of the MDR. It remains to be seen what form this proof will take for the large number of substance-based medical devices on the market.
This article was written in collaboration with Alexander Müller, who is currently working as a student trainee at the Johner Institute.
We need your feedback
Due to the implementation of Rule 21, many substance-based medical devices will be classified higher in the future, which makes the involvement of a notified body for conformity assessment necessary. When an application is submitted, the notified body will check whether the product is a medical device as defined in the MDR and whether it is correctly classified.
According to the outputs of a survey of notified bodies conducted by the European Commission, incorrect qualification and classification of medical devices is the third most common reason for rejected MDR applications. In addition, the notified bodies explicitly criticized the lack of evidence of a non-pharmacological or non-metabolic mode of action of ingredients.
We would like to ask you for your experiences in order to get a picture of the actual situation on the market. Are you a manufacturer of substance-based medical devices? Then, we look forward to your brief feedback!