Regulatory Intelligence - a core task of Regulatory Affairs?
Many companies consider regulatory intelligence so important that they create their own roles and departments for it.
This article clarifies what regulatory intelligence is, how companies can benefit from it, and where tools can provide support.
This article focuses on the medical device ecosystem, but the statements can be applied to regulatory intelligence at pharmaceutical companies almost without exception.
1. What is “Regulatory Intelligence”?
Definition: Regulatory Intelligence
Regulatory intelligence is a systematic process of collecting, analyzing, and disseminating information about regulatory requirements, policies, and guidelines that affect the development, manufacturing, distribution, and surveillance and regulation of medical devices.
Source: Johner Institute
This includes gathering and analyzing regulatory information from various sources, including regulatory agencies, industry associations, and scientific literature.
Note: Analogy CIA
"Intelligence" is not only to be interpreted as "intelligence" but also as "information" and "knowledge."
This is comparable to the CIA, the US "intelligence agency". It collects and evaluates information and passes on its findings to relevant recipients.
2. What is the goal of Regulatory Intelligence?
The goal of regulatory intelligence at manufacturers is to provide executives with the latest information on an ongoing basis. They need this to make informed decisions in the following areas:
- Deciding on medical devices to develop
- Determine which markets to bring products to and in what order
- Determine the current and future regulatory requirements in these markets
- Decide whether to participate in or object to planned regulations
- Estimate efforts, durations, and costs to meet regulatory requirements
- Ensure that the necessary staff with the required skills and competencies are in place
- Plan and organize additional resources (service providers, tools, financial resources)
Attention
Manufacturers are obliged to systematically record the regulatory requirements. ISO 13485 contains this requirement, e.g., in chapter 5.2.
3. Who benefits from Regulatory Intelligence?
Regulatory intelligence is important to a wide range of stakeholders involved in the development, manufacturing, distribution, monitoring, and regulation of medical devices. These include medical device manufacturers, regulatory affairs professionals, clinical researchers, and healthcare providers (see Tab. 1).
Role, stakeholders | Demand for regulatory intelligence |
Manufacturers | See list in section 2 |
Healthcare providers | - Decide on the purchase of medical products - Operate products in compliance with the law - Inform patients in accordance with the law - Assess safety, performance and availability of medical devices |
Authorities, notified bodies | - Prepare own employees for future requirements - Inform manufacturers about these requirements - Ensure conformity of products and manufacturers during approval and market surveillance |
Clinical researchers | - Understand regulatory requirements - Select markets for clinical investigations - Plan and conduct clinical investigations in compliance with regulatory requirements |
Legislator | - Coordinate own plans with other legislators - Model and anticipate the effects of regulation (see Regulatory Science) |
Tab. 1: Stakeholders benefiting from regulatory intelligence
4. What data is collected and where?
a) Content
In order to achieve the above objectives, all regulatory-relevant content must be collected:
- New requirements or new versions of regulatory requirements such as standards, laws, guidelines, regulations, policies, best practices, etc.
- Type of changes
- Effective date and transition periods
- Announcement of regulatory changes
- Calls for comments or the creation of regulatory-related documents
b) Sources
Sources now primarily include:
- websites and documents published there
- web-based databases
- newsletters
- online forums
- social media
Publications in printed publications such as magazines are rarer. Physical circulars and notices are the exceptions.
Some information is not official but can only be obtained through personal contacts.
5. Are there tools for Regulatory Intelligence?
For medical devices, there are services and software applications that support the collection and evaluation of regulatory information.
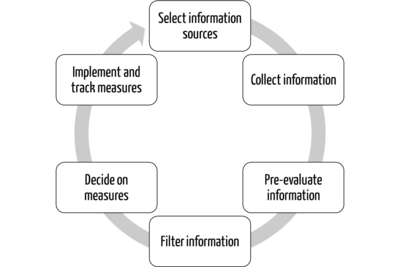
These services perform many tasks for clients (e.g., manufacturers), such as collecting, (pre)evaluating, and filtering information. They also provide support in deriving the necessary measures.
Implementing these measures is always the task of the respective client (see Tab. 2).
Task | Service provider | Client/Customer (manufacturer, authority, notified body) |
Collect information | X |
|
(Pre)evaluate information | X |
|
Filter information for client | X |
|
Decide on measures | X | X |
Implement measures | (X) | X |
Track implementation | (X) | X |
Tab. 2: Division of tasks between service provider and principal
Examples of measures include:
- Adapt standard operation precedures and other specification documents
- Perform gap analyses to verify product and organizational compliance with current versions of regulatory documents
- Update regulatory strategies (e.g., sequence of target markets)
- Inform or/and qualify employees and suppliers
Tip
Service providers like the Johner Institute help with customized services and software even to track and ensure the implementation of measures.
6. Summary and conclusion
Overall, regulatory intelligence plays a critical role in meeting the duty,
- to keep stakeholders informed of regulatory requirements and changes in the medical device industry and to
- to take the necessary action.
By providing access to regulatory information and analysis, tools help stakeholders make informed decisions about medical device development, regulatory compliance, and market access to gain a competitive advantage.
For most organizations, it makes good business sense to outsource work to service providers to benefit from "leverage" and higher-quality search results.
Support
Hundreds of manufacturers use the Regulatory Radar, part of Johner Institute's regulatory intelligence service. It monitors more than 4000 regulatory-related documents.
Contact us to learn how Regulatory Radar can save you time and money and ensure your compliance.