Medical Device Directive 93/42/EWG (MDD)
The MDD is the Medical Device Directive or 93/42/EEC. The number indicates the year of initial release (1993) and the consecutive number of directives in that year.
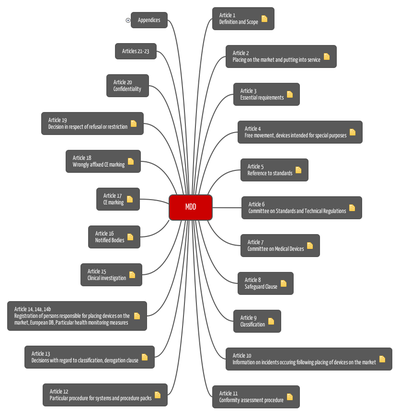
European national states must translate this directive into national law. As most of these laws directly refer back to the directive, the MDD actually defines the "essential requirements" and prerequisites to market medical devices in the European community.
Content of Medical Device Directive
The aim of the Medical Device Directive is:
- to ensure free movement of medical devices in Europe (see, eg, Articles 2 and 4), and
- to formulate EU-wide requirements for these products (see eg Article 3 and Annex I).
Past and Future of MDD 93/42/EWG
The medical device directive was introduced in 1993 and appended in 2007 by 2007/47/EC. For more than 25 year it served as the most important regulatory document in Europe.
The new Medical Devices Regulation is coming. It was released in May 2017 and will replace both, the MDD and the directive for Active Implantable Medical Devices AIMD. There is a transition period lasting between 3 and 5 years depending on the class of the device and the annex certificate issued by the notified bodies.